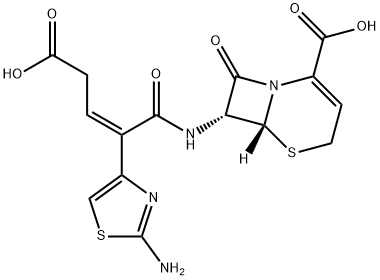
Цефтибутен
- английское имяCeftibuten
- CAS №97519-39-6
- CBNumberCB7299436
- ФормулаC15H14N4O6S2
- мольный вес410.42
- EINECS810-182-0
- номер MDLMFCD00864918
- файл Mol97519-39-6.mol
| Температура кипения | 966℃ |
| плотность | 1.75±0.1 g/cm3(Predicted) |
| RTECS | XI0367220 |
| Fp | >110°(230°F) |
| температура хранения | Keep in dark place,Sealed in dry,2-8°C |
| растворимость | Soluble in aqueous solutions. Also soluble in DMSO |
| пка | 2.99±0.50(Predicted) |
| форма | powder |
| цвет | white to beige |
| FDA UNII | IW71N46B4Y |
| Код УВД | J01DD14 |
| UNSPSC Code | 51284115 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P285:В случае недостаточной вентиляции используйте средства защиты органов дыхания.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P304+P341:В случае пожара: Риск взрыва. Покинуть опасную зону. НЕ тушить пожар в случае распространения огня на взрывчатые вещества.
P312:Обратиться за медицинской помощью при плохом самочувствии.
P342+P311:При возникновении симптомов астмы или затрудненного дыхания обратиться за медицинской помощью.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Цефтибутен химические свойства, назначение, производство
Описание
Ceftibuten is a new, once daily, orally active cephalosporin introduced as a treatment of Gram-negative bacteria-related urinarylrespiratory tract and gynecological infections. In vitro studies of 359 strains of Gram-negative bacteria demonstrated that ceftibuten was superior to cefaclor and as active or slightly more active than cefixime and cefteram.Использование
anorexic, antidepressant, inhibitor of 5HT, norepinephrine & dopamine uptakeОпределение
ChEBI: A third-generation cephalosporin antibiotic with a [(2Z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino substituent at the 7 position of the cephem skeleton. An orally-administered agent, ceftibuten is used as the dihydrate to tre t urinary-tract and respiratory-tract infections.Антимикробная активность
A semisynthetic cephalosporin formulated as the dihydrate for oral administration.It exhibits good activity against many Gram-negative bacilli, but its activity against Gram-positive cocci is very poor. It is stable to hydrolysis by the common plasmid-mediated β-lactamases, but not derepressed chromosomal enzymes .
It is rapidly and almost completely absorbed by mouth and is excreted in the urine with a half-life of 1.5–3 h. An oral dose of 400 mg achieves a peak plasma concentration of around 15 mg/L. Binding to plasma proteins is 65–77%.
Side effects mostly consist of mild gastrointestinal symptoms and mild liver function test changes. Clinical trials have mainly been conducted in urinary tract and respiratory tract infections which, despite the poor in-vitro activity against Str. pneumoniae, have shown ceftibuten to be as efficacious as comparator agents.
Общее описание
Chemical structure: ?-lactamФармакокине?тика
Ceftibuten is highly (75–90%) absorbed on oral administration, but this is decreased significantly by food. Being lipophilic and acidic, it is significantly (65%) serum protein bound. Some isomerization of the geometry of the olefinic linkage appears to take place in vivo before excretion.Клиническое использование
Ceftibuten (Cedax) is a recently introduced, chemicallynovel analog of the oximino cephalosporins in which anolefinic methylene group (C=CHCH2-) with Z stereochemistryhas replaced the syn oximino (CBNO-) group.This isosteric replacement yields a compound that retainsresistance to hydrolysis catalyzed by many β-lactamases,has enhanced chemical stability, and is orally active. Oralabsorption is rapid and nearly complete. It has the highestoral bioavailability of the third-generation cephalosporins.Ceftibuten is excreted largely unchanged in the urine andhas a half-life of about 2.5 hours. Plasma protein binding ofthis cephalosporin is estimated to be 63%.
Ceftibuten possesses excellent potency against mostmembers of the Enterobacteriaceae family, H. influenzae,Neisseria spp., and M. catarrhalis. It is not active against S.aureus or P. aeruginosa and exhibits modest antistreptococcal activity. Ceftibuten is recommended in the managementof community-acquired respiratory tract, urinary tract, andgynecological infections.
Цефтибутен запасные части и сырье
Цефтибутен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18627774460 | CHINA | 742 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +8613367258412 | China | 10319 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| +86-027-59207850 | China | 5972 | 58 |