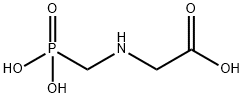
ГЛИФОСАТ
- английское имяGlyphosate
- CAS №1071-83-6
- CBNumberCB7680517
- ФормулаC3H8NO5P
- мольный вес169.07
- EINECS213-997-4
- номер MDLMFCD00055350
- файл Mol1071-83-6.mol
| Температура плавления | 230 °C (dec.) (lit.) |
| Температура кипения | 465.8±55.0 °C(Predicted) |
| плотность | 1.74 |
| Fp | 230°C |
| температура хранения | APPROX 4°C |
| растворимость | DMSO: slightly soluble; PBS (pH 7.2): slightly soluble |
| пка | 1.22±0.10(Predicted) |
| форма | solid |
| цвет | White to off-white |
| Запах | odorless |
| Растворимость в воде | 1.2 g/100 mL |
| Разложение | 230 ºC |
| Мерк | 13,4525 |
| БРН | 2045054 |
| Стабильность | Stable. Incompatible with metals, strong oxidizing agents, strong bases. May be light sensitive. |
| ИнЧИКей | XDDAORKBJWWYJS-UHFFFAOYSA-N |
| Стандарт первичной питьевой воды EPA | MCL:0.7,MCLG:0.7 |
| LogP | -2.360 (est) |
| Справочник по базе данных CAS | 1071-83-6(CAS DataBase Reference) |
| FDA 21 CFR | 165.123 |
| Рейтинг продуктов питания EWG | 3 |
| Словарь онкологических терминов NCI | asset |
| FDA UNII | 4632WW1X5A |
| Предложение 65 Список | Glyphosate |
| МАИР | 2A (Vol. 112) 2017 |
| Система регистрации веществ EPA | Glyphosate (1071-83-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.71 |
| Коды опасности | Xi,N,Xn | |||||||||
| Заявления о рисках | 41-51/53-62-37/38-36/37/38-36-22 | |||||||||
| Заявления о безопасности | 26-39-61-2-37-36 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | MC1075000 | |||||||||
| кода HS | 29319090 | |||||||||
| Банк данных об опасных веществах | 1071-83-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats, mice (mg/kg): 4873, 1568 orally (Bababunmi) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H411:Токсично для водных организмов с долгосрочными последствиями.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P362+P364:Снять всю загрязненную одежду и выстирать ее перед повторным использованием.
P391:Ликвидировать просыпания/проливы/утечки.
ГЛИФОСАТ химические свойства, назначение, производство
Описание
Glyphosate (N-(phosphonomethyl)glycine; 1071-83-6) is the active ingredient in several commercial herbicides for nonselective weed control. Glyphosate herbicides are among the world’s most widely used herbicides. Roundup?, containing the active ingredient glyphosate, was developed and introduced by Monsanto Company in 1974. Other formulations include WeatherMax, UltraMAX, Buccaneer, Razor Pro, Rodeo, and AquaMaster?. Some crops such as soybeans and cotton have been genetically engineered to be resistant to glyphosate (Roundup Ready), allowing farmers to use glyphosate as a postemergence herbicide. The United States Environmental Protection Agency (EPA) considers glyphosate to be relatively low in toxicity compared to organochlorine and organophosphate pesticides.Химические свойства
Glyphosate is a broad-spectrum, non-selective systemic herbicide. It is a colorless crystal at room temperature and is soluble in acetone, ethanol, xylene, and water. Glyphosate is used for the control of annual and perennial plants, including grasses, sedges, broadleaved weeds, and woody plants. It can be used on non-cropland as well as on many varieties of crops. Glyphosate itself is an acid, but it is commonly used in salt form, most commonly isopropylamine salt. It may also be available in acidic or trimethylsulfonium salt forms. It is generally distributed as water-soluble concentrates and powders. Glyphosate is a GUP.Использование
Glyphosate is the active ingredient in several commercial herbicides. It is a broad-spectrum systemic herbicide for various types of weeds, grasses (Poaceae), and woody plants.Общее описание
Odorless white powder. Decomposition begins at approximately 419°F (darkens). pH (1% solution in water) 2.5.Профиль реактивности
Glyphosate may react with galvanized steel or unlined steel (except stainless steel) containers to produce hydrogen gas which may form a highly combustible or explosive gas mixture. Glyphosate can react with caustic (basic) materials to liberate heat. Glyphosate is corrosive to iron.Угроза здоровью
Glyphosate is practically non-toxic if ingested, with a reported acute oral LD50 of 5600 mg/kg in the rat. The toxicities of the technical acid (glyphosate) and the formulated product (Roundup) are nearly the same. Laboratory animals, such as rats, dogs, mice, and rabbits, exposed to glyphosate for 2 years did not indicate any kind of adverse health effects.Пожароопасность
Flash point data for Glyphosate are not available; however, Glyphosate is probably combustible.Фармаколо?гия
Glyphosate is the only known inhibitor of the biosynthesis of aromatic acids that has been commercialized as a successful herbicide (1). Glyphosate acts as a competitive inhibitor of phosphoenolpyruvate, the natural substrate of the enzyme 5-enolpyruvyl-shikimate-3-phosphate (EPSP) synthase, and causes amassive accumulation of shikimate in treated plant tissue (1).Glyphosate is a nonselective herbicide, and it has been characterized as a low-risk herbicide for the evolution of herbicide resistance. A few weed species are somewhat tolerant to glyphosate, probably due to uptake or translocation mechanisms, but no plant species has sufficient resistance to glyphosate to allow its use directly on the crop as a selective herbicide. The complicated procedure used to genetically engineer the commercialized glyphosate-tolerant crops (31) would suggest that the evolution of glyphosate-resistant weeds will be a very slow process and that the level of resistance from field selection will be relatively low.
Профиль безопасности
Poison by intraperitoneal route. Moderately toxic by ingestion. Human systemic effects: arrhythmias, blood pressure lowering, body temperature increase, change in heart rate, convulsions, darrhea, fibrosing alveolitis, fibrosis, hypermoultty, respiratory depression, respiratory stimulation. Used as an herbicide. When heated to decomposition it emits very toxic fumes of NOx and POx.Возможный контакт
A potential danger to those involved in the manufacture, formulation, and application of this nonselective and nonresidual pre-emergence organophos phate herbicide. Has wide residential use in the United States for the control of weeds.Метаболический путь
The photolytic degradation of glyphosate results in the formation of glycine, (aminomethyl)phosphonic acid (AMPA), and NH3. Glyphosate undergoes nitrogen ? carbon cleavage on reaction with m- chloroperoxybenzoic acid, leading ultimately to many of the same products formed on their metabolism and environmental degradation. It is suggested that insoluble complexes of glyphosate with iron(III), copper(II), calcium, and magnesium ions are formed at near-neutral pH, a mechanism of which is the inactivation of glyphosate in contaminated groundwater.268 The bacterium degrades high levels of glyphosate, primarily by converting to AMPA. Appreciable uptake of glyphosate is observed with seedlings and leaves and to a lesser extent with culture cells in the form of non-metabolized glyphosate, with AMPA as the only detectable metabolite.Метаболизм
In soils, glyphosate is rapidly mineralized within 1 to 2 weeks, and degradation occurs under aerobic and anaerobic conditions (79). The C?P bond is relatively resistant to chemical degradation, but several bacteria, e.g., Arthrobacter (80), Pseudomonas (81), various members of the Rhizobiaceae family (82), and certain fungi (83), have been shown to metabolize glyphosate.Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz ardous material, Technical Name Required.Несовместимости
Organophosphates are susceptible to for mation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water, and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithio carbamates, isocyanates, mercaptans, nitrides, sulfides (releasing heat, toxic, and possibly flammable gases), thio sulfates, and dithionites (releasing hydrogen sulfate and oxides of sulfur). Solutions are corrosive to iron, unlined steel, and galvanized steel, forming a highly combustible or explosive gas mixture. Do not store glyphosate in contain ers made from these materials.ГЛИФОСАТ запасные части и сырье
ГЛИФОСАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18994963526 +8618994963526 |
United Kingdom | 176 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +16837774622 | China | 295 | 58 | ||
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 |