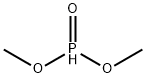
ДИМЕТИЛФОСФИТ
- английское имяDimethyl phosphite
- CAS №868-85-9
- CBNumberCB9248221
- ФормулаC2H7O3P
- мольный вес110.05
- EINECS212-783-8
- номер MDLMFCD00044633
- файл Mol868-85-9.mol
| Температура кипения | 170-171 °C(lit.) |
| плотность | 1.2 g/mL at 25 °C(lit.) |
| показатель преломления | n |
| Fp | 71 °C |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| форма | Colorless liquid with a mild odor |
| Растворимость в воде | Soluble in water. |
| Чувствительный | Moisture Sensitive |
| БРН | 1697490 |
| Стабильность | Stable. Moisture sensitive. Incompatible with water, strong oxidizing agents, acid chlorides, strong bases. |
| InChI | InChI=1S/C2H7O3P/c1-4-6(3)5-2/h6H,1-2H3 |
| ИнЧИКей | HZCDANOFLILNSA-UHFFFAOYSA-N |
| SMILES | P(=O)(OC)OC |
| Справочник по базе данных CAS | 868-85-9(CAS DataBase Reference) |
| FDA UNII | ST4TBO000H |
| МАИР | 3 (Vol. 48, 71) 1999 |
| Справочник по химии NIST | Phosphonic acid, dimethyl ester(868-85-9) |
| Система регистрации веществ EPA | Dimethyl phosphite (868-85-9) |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 21-36-10-68-52/53-43-40 | |||||||||
| Заявления о безопасности | 26-36/37-61 | |||||||||
| РИДАДР | UN 3278 6.1/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | SZ7710000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3.2 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29209013 | |||||||||
| Банк данных об опасных веществах | 868-85-9(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
ДИМЕТИЛФОСФИТ химические свойства, назначение, производство
Химические свойства
colourless liquidИспользование
- Dimethyl Phosphite(DMP)is used as a reagent in the synthesis of 4-(thiophen-2-ylmethyl)-2H-phthalazin-1-ones as potent PARP-1 inhibitors.
- It is also used as a reagent in the synthesis of estafiatin phosphonate derivatives which exhibit antibacterial and antifungal activity.
- Dimethyl Phosphite is a degradation product of the pesticides trichlorphon and malathion and may be released into the envionment following their application. It is a contaminant (approxiately 2%) in the chemical intermediate triethyl phosphite, which hydrolyses readily to dimethyl hydrogen phosphite in the presence of moist air or water.
- Dimethyl Phosphite is used as a flame retardant on Nylon 6 fibres and, in combination with guanidine and formaldehyde, to impart flame and crease resistance to cotton textiles. The compound is also used to increase fire resistance to cellulosic textiles, acrolein-grafted polyamide fibres and y-irdiated polyethylene.
- lt is used as a lubricant additive, as a chemical intermediate in the production of organophosphorous pesticides and as an adhesive.
- Dimethyl Phosphite has also been used as a stabilizer in oil and plaster and, in combination with pyroctechol, as a corrosion inhibitor on steel.
прикладной
DMP is a basic chemical which is used industrially as an intermediate. Because of its reactivityDMP participates in a large number of chemical reactions:
- Addition to oxo compounds
- Addition to oxo compounds with subsequent condensation e.g. with amines
- Oxidation with oxygen or chlorine
- Addition to alkenes
- water treatment chemicals e.g. corrosion inhibitors for cooling-water circuits (about 50 %)
- pesticides and pharmaceuticals (about 20 %)
- flame retardants and other specialities (about 15 %)
- textile finishing products (about 15 %)
Угроза здоровью
Dimethyl Phosphite (DMP) is rapidly absorbed via the oral and dermal routes. The main metabolic pathway in rodents is demethylation to monomethyl hydrogen phosphite (MMP) and further oxidation to CO2. DMP was mainly eliminated via urine and expired air. Over the studied dose range between 10 and 200 mg/kg bw and 5 x 200 mg/kg bw, respectively, only little evidence of bioaccumulation or saturation of absorption and elimination was observed. The only difference in studied toxicokinetics between rats and mice was the more rapid metabolism and elimination in mice.An inhalation LC50 value is not available, but an exposure of 7100 mg/m³ (concentration estimated based on air flow and net loss of material) over 6 hours was not lethal for rats, mice and guinea pigs. Clinical signs were observed in mice only, and included occasionally laboured respiration after approximately 2 hours of exposure and ptosis after 5 hours. The acute dermal LD50 was 681 mg/kg bw (rabbits). Signs of intoxication were depression, ptosis, labored respiration, ataxia and placidity. The acute oral LD50 values were: 3283 mg/kg bw for male rats, 3040 mg/kg bw for female rats, 2815 mg/kg bw for male mice, and between 2150 and 3160 mg/kg bw for female mice. Clinical signs were inactivity, weakness, prostration and shallow breathing at doses near to or exceeding the LD50 values. White opaque eyes were seen in male mice.
Dimethyl Phosphite is irritating to the skin and eyes of rabbits. After prolonged or repeated exposures moderate to severe irritation of skin and mucosa was observed in rats. No sensitisation studies are available.
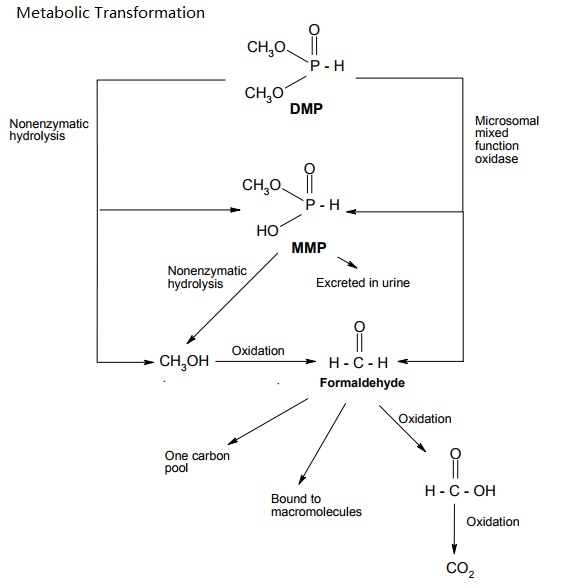
Proposed metabolic pathways of DMP in rats and mice (Nomeir and Matthews, 1997).
Профиль безопасности
Suspected carcinogen with experimental carcinogenic data. Moderately toxic by ingestion and skin contact. A skin and eye irritant. Mutation data reported. When heated to decomposition it emits toxic fumes of POxКанцерогенность
Dimethyl hydrogen phosphite was not mutagenic to several strains of Salmonella typhimurium, but it did cause sister chromatid exchanges and chromosomal aberrations in the Chinese hamster CHO line.An ACGIH threshold limit value (TLV) has not been established for dimethyl hydrogen phosphite.
ДИМЕТИЛФОСФИТ запасные части и сырье
сырьё
запасной предмет
1of3
ДИМЕТИЛФОСФИТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-86-1913198-3935 +8617331935328 |
China | 970 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 |