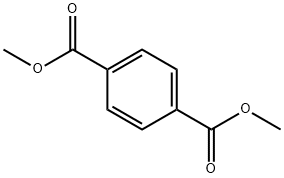
ДИМЕТИЛТЕРЕФТАЛАТ
- английское имяDimethyl terephthalate
- CAS №120-61-6
- CBNumberCB9170694
- ФормулаC10H10O4
- мольный вес194.18
- EINECS204-411-8
- номер MDLMFCD00008440
- файл Mol120-61-6.mol
| Температура плавления | 140 °C |
| Температура кипения | 288 °C |
| плотность | 1,29 g/cm3 |
| Плотность накопления | 500kg/m3 |
| плотность пара | 1.04 (vs air) |
| давление пара | 1.15 mm Hg ( 93 °C) |
| показатель преломления | 1.4752 |
| Fp | 154 °C |
| температура хранения | Store below +30°C. |
| растворимость | water: slightly soluble0.0493g/L at 20°C |
| форма | Flakes or Pellets |
| пка | 0[at 20 ℃] |
| цвет | White |
| Пределы взрываемости | 0.8-11.8%(V) |
| Растворимость в воде | It is slightly soluble in water but soluble in hot alcohol and ether. |
| Мерк | 14,9162 |
| БРН | 1107185 |
| Стабильность | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. |
| LogP | 2.25 at 25℃ |
| Справочник по базе данных CAS | 120-61-6(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | DIMETHYL TEREPHTHALATE |
| FDA UNII | IKZ2470UNV |
| Система регистрации веществ EPA | Dimethyl terephthalate (120-61-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 24/25 | |||||||||
| РИДАДР | 3256 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WZ1225000 | |||||||||
| Температура самовоспламенения | 520 °C DIN 51794 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2917 37 00 | |||||||||
| Банк данных об опасных веществах | 120-61-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 3200 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ДИМЕТИЛТЕРЕФТАЛАТ химические свойства, назначение, производство
Описание
Dimethyl terephthalate (DMT) is an organic compound with the formula C6H4(CO2CH3)2. It is the diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.Химические свойства
The empirical formula of dimethyl terephthalate (DMT) is C10H10O4. Its structural formula is 1,4-(COOCH3)2C6H4. At room temperature, exists as colorless crystals. DMT is soluble in ether and chloroform, slightly soluble in ethanol, and fairly insoluble in water (<1 g/L at 13℃).Использование
Dimethyl terephthalate is used in the production of polyesters, including polyethylene terephthalate (PET) and poly trimethylene terephthalate. It consists of benzene substituted with carboxy methyl groups (CO2CH3) at the 1 and 4 positions. Because DMT is volatile, it is an intermediate in some schemes for the recyclic of PET, e.g. from plastic bottles.Hydrogenation of DMT affords the diol cyclohexanedimethanol, which is a useful monomer.
Определение
ChEBI: Dimethyl terephthalate is a diester resulting from the formal condensation of the carboxy groups of terephthalic acid with methanol. It is a primary ingredient widely used in the manufacture of polyesters and industrial plastics. It is a methyl ester, a diester and a phthalate ester. It derives from a terephthalic acid.Подготовка
Several processes have been developed for the preparation of dimethyl terephthalate from p-xylene, but the most important proceeds as follows: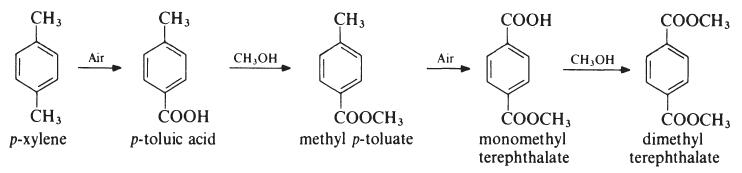
The oxidation steps are carried out in the liquid phase at about 170??C and 1.5 MPa (15 atmospheres) in the presence of a cobalt acetate or naphthenate catalyst whilst the esterifications are conducted at about 150??C. Dimethyl terephthalate may also be produced by esterification of terephthalic acid.
Методы производства
Dimethyl terephthalate (DMT) has been produced in a number of ways. Conventionally and still of commercial value is the direct esterification of terephthalic acid. Alternatively, it can be prepared by alternating oxidation and methyl-esterification steps from p-xylene via methyl-p-toluate.Общее описание
Dimethyl terephthalate appears as white solid or heated colorless liquid. Has no odor. Liquid solidifies in cool water. Solid and liquid sink in water. (USCG, 1999)Реакции воздуха и воды
When mixed with air, the vapor or dust forms very hazardous and highly reactive mixtures. . Insoluble in water.Профиль реактивности
DIMETHYL TEREPHTHALATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Can generate electrostatic charges. [Handling Chemicals Safely 1980. p. 250]. DIMETHYL TEREPHTHALATE is sensitive to heat. The molten material reacts with water due to the temperature. DIMETHYL TEREPHTHALATE is incompatible with strong oxidizers, strong acids and strong bases.Угроза здоровью
Molten DMT will cause severe burns of skin on contact.Пожароопасность
DIMETHYL TEREPHTHALATE is combustible.Профиль безопасности
Moderately toxic by intraperitoneal route. Mdly toxic by ingestion. An eye irritant. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumesКанцерогенность
In a study conducted by the NCI, DMT was not considered to be carcinogenic in rats or mice ingesting 2500 or 5000 ppm in the diet for 103 weeks.Источник
Dimethyl terephthalate is a natural product found in Hypotrachyna nepalensis, Uncaria elliptica, and other organisms with data available.Методы очистки
Purify it by recrystallisation from aqueous EtOH, MeOH or CCl4; or by zone melting. [Beilstein 6 H 843, 6 III 4250, 6 IV 3303.] .ДИМЕТИЛТЕРЕФТАЛАТ запасные части и сырье
сырьё
1of3
запасной предмет
- polyester-based liquid crystalling ionmer containing sulfonate group
- Polyester resin paint
- Моно-метил терефтала
- Polyethylene Terephthalate
- Antistatic finishing agent
1of3
ДИМЕТИЛТЕРЕФТАЛАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8615373025980 | China | 895 | 58 | ||
| +86-18186686046 +86-18186686046 |
China | 5861 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +86-16632312509 +86-16632315022 |
China | 86 | 58 | ||
| +undefined17712220823 | China | 615 | 58 |