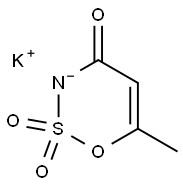
КАЛИЙ ацесульфам
- английское имяAcesulfame potassium
- CAS №55589-62-3
- CBNumberCB3224119
- ФормулаC4H4KNO4S
- мольный вес201.24
- EINECS259-715-3
- номер MDLMFCD00043833
- файл Mol55589-62-3.mol
| Температура плавления | 229-232°C (dec.) |
| Температура кипения | 210℃[at 101 325 Pa] |
| плотность | (solid) 1.81 g/cm3; d (bulk) 1.1-1.3 kg/dm3 |
| давление пара | 0.291Pa at 25℃ |
| температура хранения | Inert atmosphere,Room Temperature |
| растворимость | Soluble in water, very slightly soluble in acetone and in ethanol (96 per cent). |
| форма | Solid |
| цвет | White crystalline solid |
| Запах | odorless with sweet taste |
| Odor Type | odorless |
| Растворимость в воде | almost transparency |
| Мерк | 14,37 |
| БРН | 3637857 |
| LogP | -2.35 at 23℃ |
| Справочник по базе данных CAS | 55589-62-3(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | ACESULFAME POTASSIUM |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 23OV73Q5G9 |
| Система регистрации веществ EPA | Acesulfame-potassium (55589-62-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Заявления о рисках | 36/37/38 |
| Заявления о безопасности | 26-36/37/39 |
| WGK Германия | 1 |
| RTECS | RP4489165 |
| кода HS | 2934990002 |
| Токсичность | LD50 in rats (mg/kg): 7431 orally, 2243 i.p. (Mayer, Kemper) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
P362+P364:Снять всю загрязненную одежду и выстирать ее перед повторным использованием.
КАЛИЙ ацесульфам химические свойства, назначение, производство
Химические свойства
Acesulfame potassium occurs as a colorless to white-colored, odorless, crystalline powder with an intensely sweet taste.История
Acesulfame-K, the potassium salt of acesulfame, is a sweetener that resembles saccharin in structure and taste profile. 5,6-Dimethyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide, the first of many sweet compounds belonging to the dihydrooxathiazinone dioxide class, was discovered accidentally in 1967. From these many sweet compounds, acesulfame was chosen for commercialization. To improve water solubility, the potassium salt was made. Acesulfame-K (Sunett) was approved for dry product use in the United States in 1988 and in Canada in October, 1994. In 2003, acesulfame-K was approved as a general purposes sweetener by the FDA.Использование
'New generation', heat-stable sweetener that has not been suspected to cause cancer nor be genotoxic. Allelic variation of the Tas1r3 gene affects behavioral taste responses to this molecule, suggesting that it is a T1R3 receptor ligand.Методы производства
Acesulfame potassium is synthesized from acetoacetic acid tertbutyl ester and fluorosulfonyl isocyanate. The resulting compound is transformed to fluorosulfonyl acetoacetic acid amide, which is then cyclized in the presence of potassium hydroxide to form the oxathiazinone dioxide ring system. Because of the strong acidity of this compound, the potassium salt is produced directly.An alternative synthesis route for acesulfame potassium starts with the reaction between diketene and amidosulfonic acid. In the presence of dehydrating agents, and after neutralization with potassium hydroxide, acesulfame potassium is formed.
Имя бренда
Sunett? and Sweet One?Фармацевтические приложения
Acesulfame potassium is used as an intense sweetening agent in cosmetics, foods, beverage products, table-top sweeteners, vitamin and pharmaceutical preparations, including powder mixes, tablets, and liquid products. It is widely used as a sugar substitute in compounded formulations,and as a toothpaste sweetener.The approximate sweetening power is 180–200 times that of sucrose, similar to aspartame, about one-third as sweet as sucralose, one-half as sweet as sodium saccharin, and about 4-5 times sweeter than sodium cyclamate.It enhances flavor systems and can be used to mask some unpleasant taste characteristics.
Профиль безопасности
When heated to decompositionemits toxic fumes of SOx.Безопасность
Acesulfame potassium is widely used in beverages, cosmetics, foods, and pharmaceutical formulations, and is generally regarded as a relatively nontoxic and nonirritant material. Pharmacokinetic studies have shown that acesulfame potassium is not metabolized and is rapidly excreted unchanged in the urine. Long-term feeding studies in rats and dogs showed no evidence to suggest acesulfame potassium is mutagenic or carcinogenic.The WHO has set an acceptable daily intake for acesulfame potassium of up to 15 mg/kg body-weight.The Scientific Committee for Foods of the European Union has set a daily intake value of up to 9 mg/kg of body-weight.
LD50 (rat, IP): 2.2 g/kg
LD50 (rat, oral): 6.9–8.0 g/kg
хранилище
Acesulfame potassium possesses good stability. In the bulk form it shows no sign of decomposition at ambient temperature over many years. In aqueous solutions (pH 3.0–3.5 at 208℃) no reduction in sweetness was observed over a period of approximately 2 years. Stability at elevated temperatures is good, although some decomposition was noted following storage at 408℃ for several months. Sterilization and pasteurization do not affect the taste of acesulfame potassium.The bulk material should be stored in a well-closed container in a cool, dry place and protected from light.
Регуляторный статус
Included in the FDA Inactive Ingredients Database for oral and sublingual preparations. Included in the Canadian List of Acceptable Non-medicinal Ingredients. Accepted for use in Europe as a food additive. It is also accepted for use in certain food products in the USA and several countries in Central and South America, the Middle East, Africa, Asia, and Australia.КАЛИЙ ацесульфам запасные части и сырье
КАЛИЙ ацесульфам поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613031735486 | China | 105 | 58 | ||
| +86-13591747876 +86-13591747876 |
China | 299 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | ||
| +8617320513646 | China | 9606 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 |