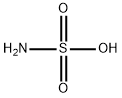
Сульфаминовая кислота
- английское имяSulfamic acid
- CAS №5329-14-6
- CBNumberCB6411280
- ФормулаH3NO3S
- мольный вес97.09
- EINECS226-218-8
- номер MDLMFCD00011603
- файл Mol5329-14-6.mol
| Температура плавления | 215-225 °C (dec.) (lit.) |
| Температура кипения | -520.47°C (estimate) |
| плотность | 2.151 g/cm3 at 25 °C |
| Плотность накопления | 600kg/m3 |
| давление пара | 0.8Pa at 20℃ |
| показатель преломления | 1.553 |
| температура хранения | Store below +30°C. |
| растворимость | water: soluble213g/L at 20°C |
| форма | Crystals or Crystalline Powder |
| пка | -8.53±0.27(Predicted) |
| цвет | White |
| РН | 1.2 (10g/l, H2O) |
| Запах | odorless |
| Растворимость в воде | 146.8 g/L (20 ºC) |
| Мерк | 14,8921 |
| Стабильность | Stable. |
| ИнЧИКей | IIACRCGMVDHOTQ-UHFFFAOYSA-N |
| LogP | 0 at 20℃ |
| Справочник по базе данных CAS | 5329-14-6(CAS DataBase Reference) |
| FDA 21 CFR | 186.1093 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SULFAMIC ACID |
| Специальный комитет по веществам GRAS | Sulfamic acid (packaging) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 9NFU33906Q |
| Справочник по химии NIST | Sulfamic acid(5329-14-6) |
| Система регистрации веществ EPA | Sulfamic acid (5329-14-6) |
| UNSPSC Code | 77101502 |
| NACRES | NA.25 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/38-52/53 | |||||||||
| Заявления о безопасности | 26-28-61-28A | |||||||||
| РИДАДР | UN 2967 8/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WO5950000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28111980 | |||||||||
| Банк данных об опасных веществах | 5329-14-6(Hazardous Substances Data) | |||||||||
| Токсичность | MLD orally in rats: 1.6 g/kg (Ambrose) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Сульфаминовая кислота химические свойства, назначение, производство
Химические свойства
Sulfamic acid is a white orthorhombic flaky crystal, odorless, non-volatile and non-hygroscopic. Soluble in water and liquid ammonia, slightly soluble in methanol, insoluble in ethanol and ether, also insoluble in carbon disulfide and liquid sulfur dioxide. Its aqueous solution has the same strong acid properties as hydrochloric acid and sulfuric acid, but its corrosiveness to metals is much lower than that of hydrochloric acid. The toxicity is extremely small, but it should not be in contact with the skin for a long time, and it should not enter the eyes.Использование
Sulfamic acid is widely used in electroplating, hard-water scale reremovers, acidic cleaning agent, chlorine stabilizers, sulfonating agents, denitrification agents, disinfectants, flame retardants, herbicides, artificial sweeteners and catalysts.Sulfamic acid is a precursor to sweet-tasting compounds. Reaction with cyclohexylamine followed by addition of NaOH gives C6H11NHSO3Na, sodium cyclamate.
Sulfamic acid is a water-soluble, moderately strong acid. An intermediate between sulfuric acid and sulfamide, it can be used as a precursor to sweet-tasting compounds, a therapeutic drug component, an acidic cleaning agent, and a catalyst for esterification.
прикладной
Sulfamic acid, the monoamide of sulfuric acid, is a strong inorganic acid. It is generally used in chemical cleaning processes like removal of nitrites, carbonate- and phosphate-containing deposits.Sulfamic acid can be used as a catalyst in:
Friedlander quinoline synthesis.
Liquid Beckmann rearrangement for the synthesis of amides from ketoximes.
The preparation of α-aminophosphonates via a three-component reaction between aldehydes, amines, and diethyl phosphite.
Определение
ChEBI: Sulfamic acid is the simplest of the sulfamic acids consisting of a single sulfur atom covalently bound by single bonds to hydroxy and amino groups and by double bonds to two oxygen atoms. It is a strong acid, readily forming sulphamate salts, which is extremely soluble in water and normally exists as the zwitterion H3N+. SO3–.Реакции
Sulfamic acid is a strong acid that reacts with many basic compounds. It is heated to above the melting point (209°C) under normal pressure to begin to decompose, and continues to be heated to above 260°C to decompose into sulfur trioxide, sulfur dioxide, nitrogen, hydrogen and water.(1) Sulfamic acid can react with metals to form transparent crystalline salts. Such as:
2H2NSO3H+Zn→Zn(SO3NH2)2+H2.
(2) Can react with metal oxides, carbonates and hydroxides:
FeO+2HSO3NH2→Fe(SO3NH2)2+H2O2
CaCO3+2HSO3NH2→Ca(SO3NH2)2+H2O+CO23
Ni(OH)2+2HSO3NH2→Ni(SO3NH2)2+H2O.
(3) Can react with nitrate or nitrite:
HNO3+HSO3NH2→H2SO4+N2O+H2O2
HNO2+HSO3NH2→H2SO4+N2+H2O.
(4) Can react with oxidants (such as potassium chlorate, hypochlorous acid, etc.):
KClO3+2HSO3NH2→2H2SO4+KCl+N2+H2O2
2HOCl+HSO3NH2→HSO3NCl2+2H2O
Общее описание
Sulfamic acid appears as a white crystalline solid. Density 2.1 g / cm3. Melting point 205°C. Combustible. Irritates skin, eyes, and mucous membranes. Low toxicity. Used to make dyes and other chemicals. It is used as a raw material for the preparation of a synthetic sweetener i.e, sodium cyclohexylsulfamate.Реакции воздуха и воды
Moderately soluble in water [Hawley].Профиль реактивности
Sulfamic acid reacts exothermically with bases. Aqueous solutions are acidic and corrosive.Опасность
Toxic by ingestion.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Токсикология
Sulfamic acid can be absorbed into the body through inhalation of its aerosols and ingestion, causing symptoms of coughing and sore throat upon inhalation; it is irritating to the skin and eyes with symptoms of redness, pain or burns; Inhaling sulfamic acid can irritate the nose and throat. High exposure to sulfamic acid can irritate the lungs. Higher exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency. sulfamic acid can cause nausea, vomiting and stomach pain. Repeated exposure to sulfamic acid may affect the liver and kidneys.Профиль безопасности
Poison by intraperitoneal route. Moderately toxic by ingestion. A human skin irritant. A corrosive irritant to skin, eyes, and mucous membranes. A substance that migrates to food from packaging materials. Violent or explosive reactions with chlorine, metal nitrates + heat, metal nitrites + heat, fuming HNO3. When heated to decomposition it emits very toxic fumes of SOx and NOx. See also SULFONATES.Возможный контакт
Sulfamic acid is used in metal and ceramic cleaning, bleaching paper pulp; and textiles metal; in acid cleaning; as a stabilizing agent for chlorine and hypochlorite in swimming pools; cooling towers; and paper mills.Перевозки
UN2967 Sulfamic acid, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Crystallise NH2SO3H from water at 70o (300mL per 25g), after filtering, by cooling a little and discarding the first batch of crystals (about 2.5g) before standing in an ice-salt mixture for 20minutes. The crystals are filtered off by suction, washed with a small quantity of ice cold water, then twice with cold EtOH and finally with Et2O. Dry it in air for 1hour, then store it in a desiccator over Mg(ClO4)2 [Butler et al. Ind Eng Chem (Anal Ed) 10 690 1938]. For the preparation of primary standard material see Pure Appl Chem 25 459 1969.Несовместимости
The aqueous solution is a strong acid. Reacts violently with strong acids (especially fuming nitric acid), bases, chlorine. Reacts slowly with water, forming ammonium bisulfate. Incompatible with ammonia, amines, isocyanates, alkylene oxides; epichlorohydrin, oxidizers.Сульфаминовая кислота запасные части и сырье
сырьё
1of2
запасной предмет
- sodium nonylphenol polyoxyethylene ether sulfate
- Nickel bis(sulphamidate)
- Disperse Red 153
- fatty alcohol ammonium sulfate
- Ацесульфам
1of6
Сульфаминовая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | ||
| +86-02785804899 +86-15071395570 |
China | 93 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86 18953170293 | China | 2930 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| +86-531-88989536 +86-15508631887 |
China | 2958 | 58 |