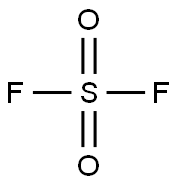
СУЛЬФУРИЛФТОРИД
- английское имяSULFURYL FLUORIDE
- CAS №2699-79-8
- CBNumberCB0165693
- ФормулаF2O2S
- мольный вес102.06
- EINECS220-281-5
- номер MDLMFCD00153272
- файл Mol2699-79-8.mol
| Температура плавления | -121,4°C |
| Температура кипения | -55,2°C |
| плотность | gas: 4.55g/L; liq; 1.7 [CRC10] |
| растворимость | slightly soluble in H2O, ethanol; soluble in toluene,ctc |
| форма | colorless gas |
| цвет | colorless |
| Растворимость в воде | mL SO2F2/100mL solvent: 4–5, H2O; 24–27, alcohol; 210–220, toluene; 136–138, CCl4, [MER06] |
| Стабильность | Stable. Reacts with water. |
| Справочник по базе данных CAS | 2699-79-8(CAS DataBase Reference) |
| FDA UNII | 64B59K7U6Q |
| Система регистрации веществ EPA | Sulfuryl fluoride (2699-79-8) |
| Коды опасности | C,N,T |
| Заявления о рисках | 23/25-36/37/38-50-48/20-23 |
| Заявления о безопасности | 23-37/39-45-61-60-63 |
| РИДАДР | 2191 |
| OEB | A |
| OEL | TWA: 5 ppm (20 mg/m3), STEL: 10 ppm (40 mg/m3) |
| Примечание об опасности | Corrosive |
| Класс опасности | 2.3 |
| Банк данных об опасных веществах | 2699-79-8(Hazardous Substances Data) |
| Токсичность | guinea pig,LD50,oral,100mg/kg (100mg/kg),Dow Chemical Company Reports. |
| ИДЛА | 200 ppm |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H400:Чрезвычайно токсично для водных организмов.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H331:Токсично при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P273:Избегать попадания в окружающую среду.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P311:Обратиться за медицинской помощью.
P314:В случае плохого самочувствия обратиться к врачу.
P391:Ликвидировать просыпания/проливы/утечки.
P403+P233:Хранить в хорошо вентилируемом месте в плотно закрытой/герметичной таре.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
СУЛЬФУРИЛФТОРИД химические свойства, назначение, производство
Химические свойства
Sulfuryl fluoride is a colorless, poisonous gas. Odorless.Использование
Sulfuryl fluoride is another promising chemical, which is widely used for control of termites, and stored-product insects. This chemical shows considerable promise and is undergoing registration procedures as replacement for methyl bromide in several countries including the United States, Great Britain, Italy, and France. The fumigant has vapour pressure of about ten times higher (13,442 mm of Hg at 25oC) than that of methyl bromide and hence it is more penetrative into treated commodities which is a significant benefit in situations where insect infestations are located deep within cracks or machinery in premises such as flour mills . The fumigant has several other advantages as a replacement for methyl bromide; it is nonreactive with structural materials and components of electronic equipment, is non flammable and has not caused problems of taint following treatment of a wide range of materials. However the sulfuryl fluoride was found to be less effective in controlling the insect eggs.Общее описание
A colorless odorless gas. Shipped as a liquefied gas under its own vapor pressure. Noncombustible. Heavier than air. Very toxic by inhalation. Contact with the unconfined liquid can cause frostbite. Prolonged exposure to heat can cause containers to rupture violently and rocket.Реакции воздуха и воды
Reacts with moist air to give corrosive acid mists that are heavier than air. Decomposes with water forming hydrofluoric acid and sulfuric acid. Reacts violently with bases [Handling Chemicals Safely p. 881 1980].Профиль реактивности
SULFURYL FLUORIDE is incompatible with water, strong oxidizing agents, alcohols, amines, and bases. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].Опасность
Toxic by inhalation. Central nervous system impairment.Угроза здоровью
TOXIC; may be fatal if inhaled or absorbed through skin. Vapors may be irritating. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.Пожароопасность
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.Сельскохозяйственное использование
Fumigant: Sulfuryl fluoride is used to fumigate closed structures and their contents such as domestic dwellings, garages, barns, storage buildings, commercial warehouses, ships in port, and railroad cars. It controls numerous insect pests including termites, powder post beetles, old house borers, bedbugs, carpet beetles, clothes moths and cockroaches, as well as rats and mice. It is also used in organic synthesis of drugs and dyes. Use pending in EU countries[ 115]. Registered for use in the U.S.Торговое название
TERMAFUME®; VIKANE®; VIKANE FUMIGANT®Профиль безопасности
Poison by ingestion. Mildly toxic by inhalation. Accidental human exposure caused nausea, vomiting, cramps, itching. May be narcotic in high concentration. Experimental reproductive effects. Can react with water, steam. When heated to decomposition it emits very toxic fumes of Fand SOx. See also FLUORIDES.Возможный контакт
Sulfuryl fluoride is used an insecticidal fumigant. It is also used in organic synthesis of drugs and dyes.Перевозки
UN2191 Sulfuryl fluoride, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, Inhalation Hazard Zone D.Несовместимости
Reacts with water and steam; at temperatures >300℃, forms strong, highly corrosive acids. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from metal hydroxides, alcohols, alkaline materials, amines, strong acids, strong bases. Fluorides form explosive gases on contact with strong acids or acid fumes.Утилизация отходов
Return refillable compressed gas cylinders to supplier. Addition of soda ash-slaked lime solution to form the corresponding sodium and calcium salt solution. This solution can be safely discharged after dilution. The precipitated calcium fluoride may be buried or added to a landfill. Small amounts could also be released directly to the atmosphere without serious harm.СУЛЬФУРИЛФТОРИД запасные части и сырье
СУЛЬФУРИЛФТОРИД поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18017061086 | CHINA | 5600 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +8615986615575 | China | 20342 | 58 | |
| +49-7246-3082843 | Germany | 1859 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 |