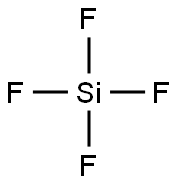
ТЕТРАФТОРСИЛАН
- английское имяSilicon Tetrafluoride
- CAS №7783-61-1
- CBNumberCB5396788
- ФормулаF4Si
- мольный вес104.08
- EINECS232-015-5
- номер MDLMFCD00040533
- файл Mol7783-61-1.mol
| Температура плавления | -90°C |
| Температура кипения | -86 °C |
| плотность | 1.66 |
| давление пара | 3517.191kPa at 25℃ |
| растворимость | reacts with H2O |
| форма | colorless gas |
| цвет | colorless |
| Удельный вес | 1.66 |
| Растворимость в воде | hydrolyzed by H2O [AIR87] |
| Гидролитическая чувствительность | 8: reacts rapidly with moisture, water, protic solvents |
| Мерк | 13,8575 |
| Справочник по базе данных CAS | 7783-61-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | K60VCI56YO |
| Система регистрации веществ EPA | Silane, tetrafluoro- (7783-61-1) |
| Коды опасности | C,T,T+ | |||||||||
| Заявления о рисках | 26-35-34-31-26/27/28-14 | |||||||||
| Заявления о безопасности | 7/9-24/25-45-36/37/39-26-23 | |||||||||
| РИДАДР | 1859 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | VW2327000 | |||||||||
| Примечание об опасности | Toxic/Corrosive | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 2.3 | |||||||||
| Банк данных об опасных веществах | 7783-61-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H300+H310+H330:Смертельно при проглатывании, при контакте с кожей или при вдыхании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P350:ПРИ ПОПАДАНИИ НА КОЖУ: Осторожно промыть большим количеством воды с мылом.
ТЕТРАФТОРСИЛАН химические свойства, назначение, производство
Химические свойства
Silicon tetrafluoride exhibits a very pungent odor. Silicon tetrachloride has a suffocating odor and is moisture sensitive.Физические свойства
Colorless gas; very pungent odor; fumes heavily in moist air; density of the gas 4.69 g/L; heavier than air, density in air 3.5 (air = 1); sublimes at -95.7°C; solidifies at -90.2°C (under pressure); critical pressure 50atm; decomposes in water forming silicic acid and hydrofluoric acid.Использование
Manufacture of fluosilicic acid, intermediate in manufacture of pure silicon, to seal water out of oil wells during drilling.Подготовка
Silicon tetrafluoride is prepared by heating silica with dilute hydrofluoric acid at high temperatures:SiO2 + 4HF → SiF4 + 2H2O
Also, the tetrafluoride may be obtained by heating the elements:
Si + 2F2 → SiF4.
Общее описание
A colorless, nonflammable, corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. Very toxic by inhalation. Vapor is heavier than air. Under prolonged exposure to heat the containers may rupture violently and rocket.Реакции воздуха и воды
Fumes in air. Decomposed exothermically by water or moisture in the air to hydrofluoric acid and silicic acid [Merck 11th ed. 1989; Handling Chemicals Safely 1980 p. 821].Профиль реактивности
When heated to decomposition SILICON TETRAFLUORIDE emits toxic fluoride fumes [Lewis, 3rd ed., 1993, p. 1138]. Reacts violently with alcohols to form HF. Attacks many metals in the presence of moisture. Reacts violently to explosively with lithium nitride [Porritt, L. J., Chem. Brit., 1979, 15, p. 282]. Mixtures with sodium are shock-sensitive explosives [Leleu, Cahiers, 1975, (79), p. 270].Опасность
Toxic by inhalation, strong irritant to mucous membranes.Угроза здоровью
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.Пожароопасность
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.Профиль безопасности
A poison. Moderately toxic by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES and HYDROFLUORIC ACIDВозможный контакт
Silicon tetrafluoride is not used in industry but may be a discharge byproduct of certain processes, including ore refining and smelting operations. It has been used in the manufacture of silane and fluosilicic acid, and pure electronic silicon.Перевозки
UN1859 Silicon tetrafluoride, Hazard Class: 2.3; Labels 2.3-Poisonous gas; 8-Corrosive material; Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Несовместимости
Water and air reactive Fluorides form explosive gases on contact with strong acids, acid fumes, and sodium. Water and air reactive. Corrosive. Fumes in air. Decomposed exothermically by water, alcohols, or moisture in the air to hydrofluoric acid and silicic acid, forming flammable and potentially explosive hydrogen gas. Attacks many metals in the presence of moisture.Утилизация отходов
For laboratory quantities, transfer into an evaporating dish containing sodium bicarbonate, spray with ammonia (6M NH4OH)/6 M-ammonium hydroxide/while stirring and then spread with crushed ice. Continue spraying with ammonia until the smoke of ammonium chloride partly subsides and add iced water while stirring. Neutralize and slowly transfer the mixture into a drain with running water.ТЕТРАФТОРСИЛАН запасные части и сырье
ТЕТРАФТОРСИЛАН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-371-66670886 | China | 19902 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-21-52699951; +8613917686115 |
China | 699 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615986615575 | China | 20342 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-37155567971 +86-13633837469 |
China | 5996 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 |