STEP A:
3-(2-nitro-4-chlorophenyl)pyruvic acid
To a cooled mixture prepared by adding 13.6 g. of
sodium to 300 ml. of ethanol is added a solution of 100
g. of 4-chloro-2-nitrotoluene and 84 g of diethyloxalate
in 150 ml of ethanol while maintaining the temperature
below 20℃. The resulting mixture is refluxed for one
hour, cooled, 400 ml. of water added and the resulting
mixture refluxed for 90 minutes. The ethanol is then
evaporated to a small volume and the resulting precipi
tate containstan and black material and the black mate
rial is mechanically separated and discarded and the
remainder (filtrate plus tan precipitate) used in the next
step. STEP B:
4-chloro-2-nitro-phenylacetic acid
The filtrate of Step A, above, is adjusted to pH 8-9
with 2 N sodium hydroxide and is heated at 35-40℃.
while adding a 3-6% aqueous hydrogen peroxide solution until samples no longer turn dark on treatment with
2N sodium hydroxide. The tan precipitate is suspended
in 1500 ml of water, adjusted to pH 8-9 and similarly
treated with aqueous hydrogen peroxide. The combined
reaction mixtures are then acidified with concentrated
hydrochloride acid to obtain a precipitate which are re
covered by filtration, washed 3 times with water and
dried in vacuo. The crude product (mp 145-150° C) is
recrystallized from ether to obtain 4-chloro-2-nitro
phenylacetic acid, m.p. 156-159℃. STEP C: The product of Step B, above, is subjected to reductive
cyclization analogously to Step B of Example 1 to
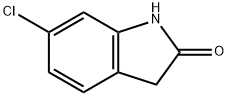
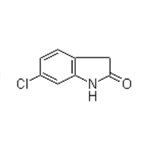
obtain 6-chloro-oxindole, m.p. 185-189℃.
Off-white to tan crystalline powder
6-Chlorooxindole is a useful research chemical used in the design of potent non-peptide MDM2 inhibitors.
ChEBI: 6-Chlorooxindole is a member of indoles.
6-chlorooxyindole, also known as 6-chloro-2-indolone, is an intermediate of Ziprasidone hydrochloride. Ziprasidone is an atypical broad-spectrum antipsychotic developed by Pfizer for the treatment of schizophrenia with a strong affinity for serotonin and dopamine receptor antagonists, particularly the 5-HTA2/DAD2 receptor.