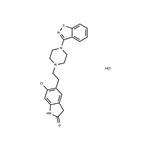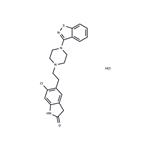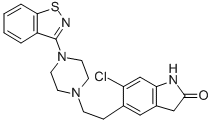
Ziprasidone
- Product NameZiprasidone
- CAS122883-93-6
- MFC21H21ClN4OS
- MW412.943
- EINECS602-903-0
- MOL File122883-93-6.mol
Chemical Properties
| Melting point | >300 °C |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO > 10 mM |
| form | Powder |
| Stability | Hygroscopic |
Usage And Synthesis
Both p.o. and i.m. formulations of ziprasidone were launched in Sweden for the
treatment of schizophrenia and agitated psychoses. It is the sixth marketed atypical
antipsychotic after clozapine, risperidone, olanzapine, sertindole and quetiapine. The
synthesis of ziprasidone involves a novel one-step process for the preparation of 3-(1-
piperazinyl)-1,2-benzisothiazole followed by coupling with a chlorooxindole fragment.
Ziprasidone is a very potent 5-HT2A/D2 antagonist with a ratio of about 11 in favor of the
serotonin receptor. It also shows very high 5-HT2c antagonistic activity, high 5-HT1A
agonistic and 5-HT1D antagonistic activity, as well as moderate antagonism of α1 and H1
receptors and moderate norepinephrine and serotonin reuptake inhibition. Its complex
binding profile for serotonin and dopamine receptors resulted during clinical trials in high
antipsychotic efficacy with low extrapyramidal side effects and also in antidepressive
action with low propensity for weight gain in opposition to other atypical and typical
neuroleptics. An intramuscular formulation of ziprasidone was demonstrated to be superior
to haloperidol, a conventional neuroleptic, for the short-term treatment of agitation in
acutely psychotic patients. When administered orally in the fed state, this well-tolerated
agent which strongly binds to plasma proteins shows a bioavailability of about 60% which
is almost 2 fold greater than in the fasted state. It is transformed into 4 circulating major
metabolites by different enzyme systems. The small QTc prolongation observed with
ziprasidone was found to be comparable to other antipsychotic drugs and it is considered
to be without significant risk.
Ziprasidone (Geodon, Zeldox) was the fifth atypical antipsychotic to gain FDA approval. In the United States, Ziprasidone is approved for the treatment of schizophrenia, and the intramuscular injection form of ziprasidone is approved for acute agitation i
Preparation of 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-
1,3-dihydro-2H-indol-2-one
A 20-gallon glass lined tank, under a nitrogen atmosphere, was charged with 33.5 liters of water and 9.4 kilograms (kg) of sodium carbonate (dense, 89.1 moles, 3.4 eq.). The resulting mixture was stirred to give a solution. To the solution 6.4 kg of 2-chloroethyl-6-chloro-oxindole (27.8 moles, 1.06 eq.) was charged, followed by 6.7 kg of 3-piperazinyl-1,2-benzisothiazole hydrochloride (26.2 moles, 1.0 eq.). This was stirred and heated to reflux (100°C). After 11 hours the reaction was sampled for high pressure liquid chromatography (HPLC) assay. The reflux was continued for another 2 hours then the reaction was cooled to 25°C and the slurry stirred for 1 hour. The product was observed and found to be essentially free from lumps and gummy matter. The product was collected by filtration. A 14 liter water was added to the tank and cooled to 12°C and then used to wash the product. The cake was pulled as dry as possible, and the product was returned to the tank along with 40 liters of isopropyl alcohol (IPO). This was cooled and then stirred for 2 hours and the product was collected by filtration. The cake was washed with 13.4 liters of fresh IPO, then dried under vacuum at 30° to 40°C. After drying, 17.3 kg of the title compound was obtained. This was in excess of the theoretical weight yield due to some residual carbonate in the crude product.
Recrystallization of 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6- chloro-1,3-dihydro-2H-indol-2-one
To a clean and dry 100-gallon glass lined tank was charged 9.0 kg of the material obtained above and 86 gallons of tetrahydrofuran (THF). The slurry was heated to reflux and held for 1 hour. The hazy solution was then filtered through a 14" sparkler precoated with filter aid and backed with a Fulflo filter to a clean, dry, and "spec free" glass-lined tank on a lower level. The batch was concentrated by vacuum distillation. Another 8.3 kg of the material obtained in above was dissolved in 83 gallons of THF in the upper tank. This was filtered to the lower tank. The tank lines and sparkler were rinsed with 10 gallons of THF. The batch was concentrated to about 22 gallons, then cooled to 5°C and stirred for 1 hour. The product was collected by filtration. Then 20 gallons of fresh IPO were cooled in the tank and used to rinse the product cake. The product was collected and dried under vacuum at 45°C; yielding 9.05 kg of product (83.8% yield for the coupling and recrystallization. The product matched the spectra of a standard NMR and showed the correct retention time by HPLC with 99.7% assay. Another way for preparation of 5-(2-(4-(1,2-benzisothiazol-3-yl)-piperazinyl)ethyl)-6-chloro-1,3-dihydro-2-H- indol-2-one.
A clean and dry 20-gallon glass lined tank was charged with 19 L of water and 4.44 kg of sodium carbonate, after the carbonate had dissolved 4.29 kg (17.5 moles) of 5-(2-chloroethyl)-6-chloro-oxindole and 3.62 kg (16.5 moles) of 1- (1,2-benzisothiazol-3-yl)piperazine were added. The aqueous slurry was heated to reflux and the temperature maintained for 14 hours. When the reaction was complete the solution was cooled to 20°C and filtered. The wet product was reslurried in 23 L of isopropyl alcohol at room temperature for 2 hours. The product was collected by filtration on 2 large Buchner funnels, each was washed with 3.4 L of fresh isopropyl alcohol. The product was vacuum dried at 30° to 40°C. until no isopropyl alcohol remained, giving 5.89 kg (86.4% yield) of the desired free base which matched a standard sample by high performance liquid chromatography (HPLC).
A clean and dry 20-gallon reactor was charged with 17.4 gallons of deionized water and 4.44 L of concentrated hydrochloric acid, to give a 0.77 M solution. To the solution was added 4.44 kg of the anhydrous 5-(2-(4-(1,2- benzisothiazol-yl)-1-piperazinyl)-ethyl)-6-chloro-1,3-dihydro-2H-indol-2-one free base. The slurry was warmed to 65°C and held for 18 hours. The slurry was cooled to room temperature. The product was filtered and washed with 2x5-gallon portions of deionized water, and then air dried at 50°C for 30 hours. The dried product contained 4.4% water and the x-ray diffraction method confirmed that the desired product was obtained.
A 20-gallon glass lined tank, under a nitrogen atmosphere, was charged with 33.5 liters of water and 9.4 kilograms (kg) of sodium carbonate (dense, 89.1 moles, 3.4 eq.). The resulting mixture was stirred to give a solution. To the solution 6.4 kg of 2-chloroethyl-6-chloro-oxindole (27.8 moles, 1.06 eq.) was charged, followed by 6.7 kg of 3-piperazinyl-1,2-benzisothiazole hydrochloride (26.2 moles, 1.0 eq.). This was stirred and heated to reflux (100°C). After 11 hours the reaction was sampled for high pressure liquid chromatography (HPLC) assay. The reflux was continued for another 2 hours then the reaction was cooled to 25°C and the slurry stirred for 1 hour. The product was observed and found to be essentially free from lumps and gummy matter. The product was collected by filtration. A 14 liter water was added to the tank and cooled to 12°C and then used to wash the product. The cake was pulled as dry as possible, and the product was returned to the tank along with 40 liters of isopropyl alcohol (IPO). This was cooled and then stirred for 2 hours and the product was collected by filtration. The cake was washed with 13.4 liters of fresh IPO, then dried under vacuum at 30° to 40°C. After drying, 17.3 kg of the title compound was obtained. This was in excess of the theoretical weight yield due to some residual carbonate in the crude product.
Recrystallization of 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6- chloro-1,3-dihydro-2H-indol-2-one
To a clean and dry 100-gallon glass lined tank was charged 9.0 kg of the material obtained above and 86 gallons of tetrahydrofuran (THF). The slurry was heated to reflux and held for 1 hour. The hazy solution was then filtered through a 14" sparkler precoated with filter aid and backed with a Fulflo filter to a clean, dry, and "spec free" glass-lined tank on a lower level. The batch was concentrated by vacuum distillation. Another 8.3 kg of the material obtained in above was dissolved in 83 gallons of THF in the upper tank. This was filtered to the lower tank. The tank lines and sparkler were rinsed with 10 gallons of THF. The batch was concentrated to about 22 gallons, then cooled to 5°C and stirred for 1 hour. The product was collected by filtration. Then 20 gallons of fresh IPO were cooled in the tank and used to rinse the product cake. The product was collected and dried under vacuum at 45°C; yielding 9.05 kg of product (83.8% yield for the coupling and recrystallization. The product matched the spectra of a standard NMR and showed the correct retention time by HPLC with 99.7% assay. Another way for preparation of 5-(2-(4-(1,2-benzisothiazol-3-yl)-piperazinyl)ethyl)-6-chloro-1,3-dihydro-2-H- indol-2-one.
A clean and dry 20-gallon glass lined tank was charged with 19 L of water and 4.44 kg of sodium carbonate, after the carbonate had dissolved 4.29 kg (17.5 moles) of 5-(2-chloroethyl)-6-chloro-oxindole and 3.62 kg (16.5 moles) of 1- (1,2-benzisothiazol-3-yl)piperazine were added. The aqueous slurry was heated to reflux and the temperature maintained for 14 hours. When the reaction was complete the solution was cooled to 20°C and filtered. The wet product was reslurried in 23 L of isopropyl alcohol at room temperature for 2 hours. The product was collected by filtration on 2 large Buchner funnels, each was washed with 3.4 L of fresh isopropyl alcohol. The product was vacuum dried at 30° to 40°C. until no isopropyl alcohol remained, giving 5.89 kg (86.4% yield) of the desired free base which matched a standard sample by high performance liquid chromatography (HPLC).
A clean and dry 20-gallon reactor was charged with 17.4 gallons of deionized water and 4.44 L of concentrated hydrochloric acid, to give a 0.77 M solution. To the solution was added 4.44 kg of the anhydrous 5-(2-(4-(1,2- benzisothiazol-yl)-1-piperazinyl)-ethyl)-6-chloro-1,3-dihydro-2H-indol-2-one free base. The slurry was warmed to 65°C and held for 18 hours. The slurry was cooled to room temperature. The product was filtered and washed with 2x5-gallon portions of deionized water, and then air dried at 50°C for 30 hours. The dried product contained 4.4% water and the x-ray diffraction method confirmed that the desired product was obtained.
H-Indol-2-one, 5-(2-(4-(1,2-benzisothiazol-3-yl)-1-
piperazinyl)ethyl)-6-chloro-1,3-dihydro-, monohydrochloride monohydrate
A Certified Spiking Solution?; applicable to use in forensic drug analysis or calibrator preparation by LC/MS or GC/MS. Ziprasidone is sold under the trade names Geodon? and Zeldox for treatment of schizophrenia and bipolar disorder. Recent studies have suggested that Ziprasidone may increase the risk of Type II diabetes and hyperglycemia.
Preparation Products And Raw materials
Ziprasidone manufacturers
Related Product Information
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine