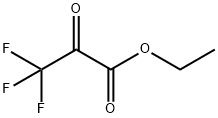
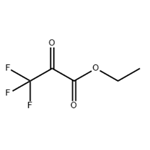
Ethyl 3,3,3-trifluoropyruvate may be used in the preparation of N-heteroaryl(trifluoromethyl)hydroxyalkanoic acid esters.
Ethyl Trifluoropyruvate is used as a reagent in the preparation of potential biologically active compounds such as in the highly enantioselective organocatalytic hydroxyalkylation of indoles .
Ethyl trifluoropyruvate acts as a building block in synthetic chemistry for the synthesis of fluorine containing compounds. It is also used as a reagent for the preparation of potential biologically active compounds like highly enantioselective organocatalytic hydroxyalkylation of indoles.
Ethyl 3,3,3-trifluoropyruvate is a trifluoromethylated compound. Enantioselective Friedel–Crafts alkylation of simple phenols and indoles with ethyl 3,3,3-trifluoropyruvate under different reaction conditions have been reported.
To 58 mL (1.0 mL/mmol) of acetonitrile, 10 g (58 mmol, 1.0 eq) of ethyl 3,3,3-trifluorolactate was added and dissolved therein.Furthermore, 11 g (67 mmol, 1.2 eq) of NaClO.5H2O was added, followed by stirring at 20° C. for 30 minutes.As the reaction-terminated liquid was analyzed by 19F-NMR, conversion was 100%, and selectivity was 98%.To the reaction-terminated liquid, 0.38 g (1.5 mmol, 0.026 eq) of sodium thiosulfate pentahydrate was added, followed by stirring, thereby quenching the remaining oxidation agent.Furthermore, 0.33 g (3.9 mmol, 0.067 eq) of sodium hydrogencarbonate and 10 g (70 mmol, 1.2 eq) of sodium sulfate were added, followed by stirring and then removing solid matter by filtration.As the filtrate was quantified with internal standard method (internal standard substance: α,α,α-trifluorotoluene) by 19F-NMR, Ethyl trifluoropyruvate was contained by 56 mmol (quantitative yield: 97%).By a simple distillation (up to 48° C./0.5 kPa) of the filtrate, 7.6 g of Ethyl trifluoropyruvate was obtained. 19F-NMR purity was 99% (40 mmol), and the total yield was 69%.
[1] Patent: US2018/50976, 2018, A1. Location in patent: Paragraph 0143; 0144; 0145; 0146; 0147; 0148; 0149; 0150