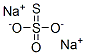Sodium thiosulphate can also be used as a complexing agent and plays an important role in the separation and purification of metal ions. For example, in silver salt photography, sodium thiosulphate can coordinate with silver ions to form a relatively stable complex, which is then reduced to silver hydroxide and precipitated.
Sodium thiosulphate is a commonly used chemical reagent with various functions, such as reducing agent, complexing agent, decolorizing agent, etc.
Sodium thiosulphate is also widely used in the decolorization process of dyes and pigments. Certain materials (such as wood, paper, etc.) develop yellow pigments during mold and aging, which can affect their appearance. Sodium thiosulphate can reduce these yellow pigments and remove them. Therefore, it is widely used in cultural preservation, food processing and cosmetics industries.
Properties and Applications
As an effective reducing agent, sodium thiosulphate is widely used in many industrial and production processes. It often acts as a reducing agent in reactions with silver oxide, reducing silver oxide to metallic silver. In addition, it is used as a reducing agent in photographic printing to reduce exposed silver salts into visible silver shadows.