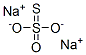Medical uses of Sodium thiosulfate
Sodium thiosulfate (STS) is an industrial chemical which also has a long medical history. It was originally used as an intravenous medication for metal poisoning. It has since been approved for the treatment of certain rare medical conditions. These include cyanide poisoning, calciphylaxis, and cisplatin toxicity. In vitro assays have demonstrated that it is an anti-inflammatory and neuroprotective agent. It therefore has potential for treating neurodegenerative diseases such as Alzheimer disease and Parkinson disease. NaSH has similar properties and is somewhat more powerful than STS in these in vitro assays. However STS has already been approved as an orally available treatment. STS may therefore be a readily available candidate for treating neurodegenerative disorders such as Alzheimer disease and Parkinson disease.

Background
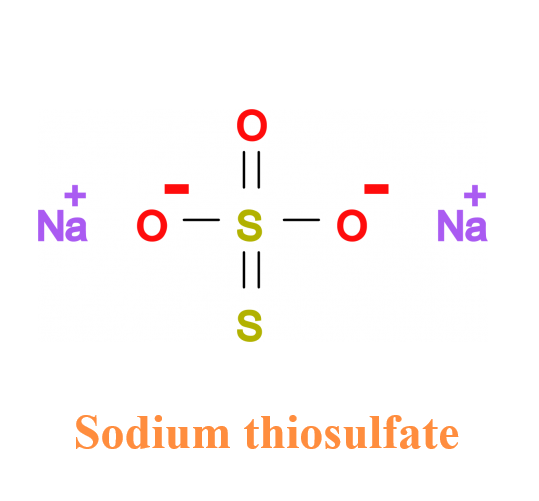
Sodium thiosulphate (Na2S2O3, STS) is an industrial compound which has a long history of medical use. Since it is employed as a food preservative, the general population is widely exposed to this non-toxic compound. For example, it is typically added to table salt at less than 0.1% and to alcoholic beverages at less than 0.0005%. It is generally available as a non-prescription oral product.
One of the early medical uses of STS was in the successful treatment of arsenic, lead, mercury and bismuth poisoning1. Halliday and Sutherland2 described using intravenous STS in a moribund case of arsenic poisoning. There was an immediate response with eventual full recovery. Shiels3 described the use of STS to treat chronic lead poisoning at the Mount Ina mine in Australia. It was then the largest single lead mine in the world. There were dozens of lead poisoning cases being diagnosed each year. Many such cases were successfully treated with intravenous injections of STS. Bertin et al.4 reviewed the outcome of treating arsenic toxicosis in cattle. The condition is typically fatal but a 50% survival rate was achieved with administration of intravenous fluids and STS. In these conditions, oral administration was ineffective. The lack of toxicity after intravenous administration emphasizes its safety. The ineffectiveness of oral administration suggests that relatively high amounts are required to treat overdoses of poisonous elements.
Contemporary Uses
STS has been approved to treat some rare medical conditions. Calciphylaxis is the most prominent. It is a potentially fatal condition resulting from kidney failure, often of unknown cause. Thrombotic lesions develop, especially in the skin. Hopeful results have been obtained through the use of intravenous STS5. It has also been directly applied to dermal lesions in doses of 250 mg/ml with resolution of the lesions over a period of weeks6. The success of these treatments is believed to be multifactorial. STS is known to be an anti-calcification agent with vasodilatory and antioxidant properties. Another STS use is to protect against cisplatin toxicity. Cisplatin is one of the most widely used agents to treat solid tumours. It has adverse effects on renal, neurological, gastrointestinal and hematological systems. Toxic overdoses are common7. To avoid this problem, STS has been successfully administered with cisplatin8. Efficacy is thought to be related to STS binding to free platinum.
Cyanide poisoning is yet another condition where STS has a treatment role. Cyanide poisoning is uncommon but deadly. It can occur in numerous situations. Examples are fires, pest control programs, and gold mining. Combinations of STS and hydroxycobalamin have proved to be effective9,10. The United States has a standard cyanide antidote kit which first uses a small inhaled dose of amyl nitrite, followed by intravenous sodium nitrite, and finally by intravenous sodium thiosulfate.
STS has shown some promise in treating other conditions. Recently STS was demonstrated to function as an anti-inflammatory agent11. For example, in acute liver failure induced in mice by lipopolysaccharide (LPS) or LPS/D-Galactosamine, the survival rate was improved by hydrogen sulfide and STS12,13. This at least is partially due to their anti-oxidative functions. STS reacts with GSSG (oxidized glutathione) to produce reduced glutathione in the presence of hydroxyl radicals or peroxides. In addition, STS has a potential to produce hydrogen sulfide by reaction with trans-sulfuration enzymes14-16.
Future Possibilities
The greatest potential uses of STS are yet to be tested. We and others have shown it be an anti-inflammatory agent. Chronic neuroinflammation is closely associated with the pathogenesis of several neurodegenerative diseases, including Alzheimer disease (AD)17,18 and Parkinson disease (PD)19-22. There are now more than 20 published epidemiological studies showing that people known to be taking anti-inflammatory agents, or known to be suffering from conditions such as arthritis for which such agents are routinely used, have a considerably reduced risk of developing AD17. The evidence that a chronic in?ammatory reaction may be contributing to neuronal death is not as strong in PD as it is in AD, but there is some evidence that antiin?ammatory agents may have a protective effect23.
To illustrate the potential of STS as a neuroprotective agent, we studied its effects as well as those of NaSH as inhibitors of SH-SY5Y neuronal cell death induced by inflammatory agents. These toxins were contained in supernatants from LPS and interferon-γ (IFNγ)-activated glial cells24. When human microglia and astrocytes are activated by LPS/IFNγ or IFNγ, they release materials that are toxic to differentiated SH-SY5Y cells. We used two cell viability assays to investigate glial-mediated neurotoxicity and anti-inflammatory effects of STS and NaSH. These were MTT to measure cell survival, and its inverse lactic acid dehydrogenase, to assess cell death. We also employed western blotting to examine the activation of intracellular inflammatory pathways.
We found that STS increases H2S and GSH expression in human microglia and astrocytes. When the glial cells were treated with NaSH or STS, there was a significant enhancement of neuroprotection. The effect was concentration-dependent and incubation time-dependent. Such treatment reduced the release of TNFα and IL-6, and also attenuated activation of P38 MAPK and NFκB proteins. The compounds tested were not harmful when applied directly to all the cell types. Although NaSH was somewhat more powerful than STS in these in vitro assays, STS has already been approved as an orally available treatment. STS may therefore be a candidate for treating neurodegenerative disorders that have a prominent neuroinflammatory component. Both agents appear to be potentially suitable as pharmaceutical drugs in conditions such as Alzheimer disease and Parkinson disease.
What is important is that STS is an approved agent while NaSH must go through many regulatory steps before it could be approved. For the present, emphasis should be on STS as a potential broad spectrum therapeutic agent.
Recent biomarker studies indicate that AD onset commences as much as 10–15 years before clinical symptoms become manifest25. The most relevant ones are CSF Abeta 42 as a differential marker25, and positron emission tomography (PET) with Pittsburgh compound B as an integral biomarker26. If simple methods of preclinical diagnosis can be achieved, a 10-year window may exist for measures to be instituted that will prevent, delay, or ameliorate any cognitive decline or clinical signs of the disease. A similar period of grace undoubtedly exists for PD since well over half of the substantial nigra neurons are lost before any clinical signs develop27.
Conducting a meaningful clinical trial of STS in AD or PD would take years and cost multimillions of dollars. This is unlikely to occur since STS is already available and recovering the cost would be a problem for a pharmaceutical company. The same is true of NSAIDs. The upside is that individuals, known to be at risk, or suspected of being at risk, can treat themselves with both these agents at a trivial cost.
Acknowledgment
The authors are grateful to Dr. Rick Sawaya (ricksawaya@gmail.com) for his advice and inspiration for these experiments. This research was supported by donations from the Angela E. Lyson Estate and other British Columbians. The authors declare no conflict of interest.
References
1.Dennie WL, McBride CC. Treatment of arsphenamine dermatitis and certain other metallic poisonings. Arch Dermatology and Syphilology. 1923; V11. 63.
2.Halliday HM, Sutherland CE. Arsenical poisoning treated by sodium thiosulphate. British Med J. 1925; 1. 467.
3.Shiels DO. The treatment of lead poisoning by the intravenous administration of sodium thiosulphate. Med J Australia. 1952:879-883.
4.Bertin FR, Baseler LJ, Wilson CR, Kritchevsky JE, Taylor SD. Arsenic toxicosis in cattle: meta-analysis of 156 cases. J Vet Intern Met. 2013; 27(4):977-981.
5.Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010; 23(3):258-262.
6.Strazzula L, Nigwekar SU, Steele D, Tsiaras W, Sise M, Bis S, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatology. 2013; 149(8):946-949.
7.Tsang RY, Al-Fayea T, Au HJ. Cisplatin overdose: toxicities and management. Drug Saf. 2009; 32(12):1109-1122.
8.Pfeifle CE, Howell SB, Felthouse RD, et al. High-dose cisplatin with sodium thiosulfate protection. J Clin Oncol. 1985; 3(2):237-44.
9.Reade MC, Davies SR, Morley PT,, et al. Review article: management of cyanide poisoning. Emer Med Australas 2012; 24(3):225-238.
10.Petrokovics I, Budai M, Kovacs K, Thompson DE. Past, present and future of cyanide antagonism research: From the early remedies to the current therapies. World J Methodol 2015; 5(2):88-100.
11.Sakaguchi M, Marutani E, Shin HS, et al. Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology 2014; 121(6):1248-1257.
12.Tokuda K, Kida K, Marutani E, et al. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012; 17(1):11-21.
13.Shirozu K, Tokuda K, Marutani E, Lefer D, Wang R, Ichinose F. Cystathionine γ-lyase deficiency protects mice from galactosamine/lipopolysaccharide-induced acute liver failure. Antioxid Redox Signal. 2014; 20(2):204-216.
14.Sen U, Vacek TP, Hughes WM, Kumar M, Moshal KS, Tyagi N, et al. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology. 2008; 82(3) :201-213.
15.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, et al. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009; 297(2): 410-419.
16.Bijarnia RK, Bachtler M, Chandak PG, van Goor H, Pasch A. Sodium thiosulfate ameliorates oxidative stress and preserves renal function in hyperoxaluric rats. PLoS One 2015;10(4): e0124881.
17.McGeer PL, Schulzer M, McGeer EG. Arthritis and antiinflammatory agents as negative risk factors for Alzheimer disease: A review of seventeen epidemiological studies, Neurology. 1996; 47:425-432.
18.Monheim AE. Oxidant/Antioxidant imbalance and the risk of Alzheimer’s disease. Curr Alzheimer Res. 2015; 12(4):335-349.
19.Smeyne M, Smeyne R. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 2013; 62:13-25.
20.Koppula S, Kumar H, More SV, et al. Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. Int J Mol Sci 2012; 13(8):10608-29.
21.Asanuma M, Miyazaki I. Nonsteroidal anti-inflammatory drugs in experimental models and Parkinson’s disease. Curr Pharm Des. 2008; 14(14):1428-1434.
22.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150(8):963-976.
23.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2006; 69(19):1836-1842.
24.Lee M, McGeer EG, McGeer PL. Sodium thiosulfate attenuates glial-mediated neuroinflammation in degenerative neurological diseases. J Neuroinflammation. 2016; 13: 32.
25.Buchhave P, Minthon L, Zetterberg H et al. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012; 69(1): 98–106.
26.Cohen AD. Using Pittsburgh compound B for PET imaging across the Alzheimer’s disease spectrum. Technology and Innovation. 2016: 51-61.
27.McGeer EG, McGeer PL, Suzuki J. Aging and extrapyramidal function. Arch Neurol. 1977; 34:33-35.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium thiosulfate manufacturers

US $5.00-0.50/KG2025-06-11
- CAS:
- 7772-98-7
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $100.00/kg2025-04-21
- CAS:
- 7772-98-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt