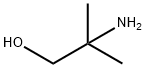
Applications of 2-Amino-2-methyl-1-propanol
2-amino-2-methyl-1-propanol((CH3)2C(NH2)CH2OH,AMP) appears as a clear light colored liquid. 2-amino-2-methyl-1-propanol is insoluble in water and about the same density as water. Flash point 172°F. 2-amino-2-methyl-1-propanol is used to make other chemicals. 2-amino-2-methyl-1-propanol is an organic compound with both amine and alcohol substituents. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
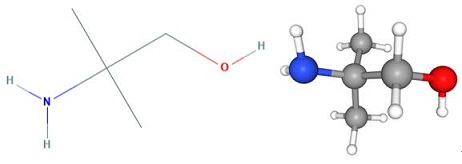
Fig 1. Chemical structure formula and three-dimensional structure of 2-Amino-2-methyl-1-propanol
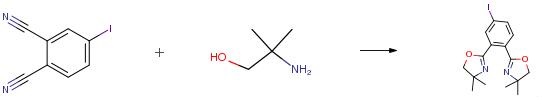
2-Amino-2-methyl-1-propanol is used for the preparation of buffer solution and in cosmetics. 2-Amino-2-methyl-1-propanol is also used in ATR-FTIR spectroscopic investigation of the carbon monoxide absorption characteristics of a series of heterocyclic diamines[1]. 2-Amino-2-methyl-1-propanol, a sterically hindered primary amine, has been studied extensively and is considered as a promising candidate for CO2 capture due to its excellent characteristics, including high absorption capacity, high degradation resistance and low regeneration energy[2]. Particularly, 2-Amino-2-methyl-1-propanol is often used as a regulatory reagent to improve the CO2 capture performance of absorbents. 2-Amino-2-methyl-1-propanol is an aminoalcohol structurally related to amino acids. The conformational study of amino acids and corresponding prototypes for the human organism is very useful, since their geometry is expected to impact the reactivity and biological activity. The conformational isomerism in a variety of amino acids has been reported to be due to steric and hyperconjugative effects rather than hydrogen bond[3]. Thus, 2-Amino-2-methyl-1-propanol is reported in this work to evaluate the role of the hydrogen bond, as well as of hyperconjugation and Lewis-type interactions, on the conformational isomerism of this compound.
Toxicity: Causes severe irritation. Inhalation may be fatal as a result of spasm, inflammation, and edema of laryns and bronchi, chemical pneumonitis, and pulmonary edema. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting.
References
[1] Halim, H. N. A.; Shariff, A. M.; Bustam, M. A. High pressure CO2 absorption from natural gas using piperazine promoted 2-amino-2-methyl-1-propanol in a packed absorption column. Sep. Purif. Technol. 2015, 152, 87-93.
[2] Khan, Anoar Ali, Halder, G.N, Saha, A.K. Carbon dioxide capture characteristics from flue gas using aqueous 2-amino-2-methyl-1-propanol (AMP) and monoethanolamine (MEA) solutions in packed bed absorption and regeneration columns[J]. International Journal of Greenhouse Gas Control, 32:15-23.
[3] Rodrigo A. Cormanich, Lucas C. Ducati, Roberto Rittner. Are hydrogen bonds responsible for glycine conformational preferences?[J]. 387(1-3):85-91.
You may like
Related articles And Qustion
Lastest Price from 2-Amino-2-methyl-1-propanol manufacturers

US $0.00/kg2025-09-24
- CAS:
- 124-68-5
- Min. Order:
- 1kg
- Purity:
- 95%min
- Supply Ability:
- 20tons
US $1.00-4.00/KG2025-09-11
- CAS:
- 124-68-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG