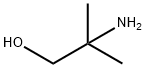
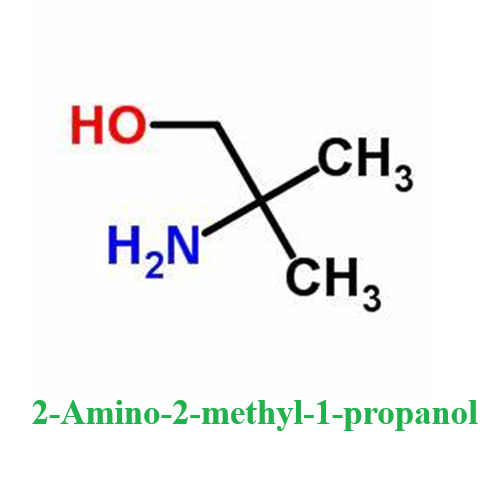
2-Amino-2-methyl-1-propanol- Reaction / Application on synthetic works
2-Amino-2-methyl-1-propanol is an important organic intermediate (building block) to synthetize substituted amino products.
The following example is about its application on the synthesis of bis(oxazoline) ligand [1].
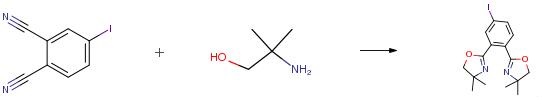
A solution of 4-iodophthalonitrile (4.0 mmol) and zinc triflate (5.0 mol percent, 0.2 mmol) in dried chlorobenzene (30 mL) was stirred at room temperature for 15 minutes. A solution of 2-amino-2-methyl-1-propanol (8.0 mmol) in dry chlorobenzene (5 mL) was slowly added. The temperature was raised to 135° C. and the reaction mixture was refluxed for 24 hours. The solvent was removed using a rotary evaporator. The crude product was dissolved in 30 mL of dichloromethane and extracted twice with distilled water (2×20.0 mL). The aqueous layer was then separated and the combined organic layers were dried with anhydrous sodium sulfate. The dichloromethane was removed was removed using a rotary evaporator to obtain the impure product, which was then purified using silica gel column chromatography with dichloromethane-ether (4:1) as eluent.
The following example is about its application on the synthesis of oxazolidines [2].
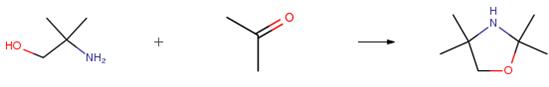
Amino-2-methylpropanol (4.89 g, 55 mmol, 8.5 eq),6.50 mmol of carbonyl compound (1 eq), and 50 mg of H2-activated clay are placed in an autoclave (100 cm3).The sealed reactor is heated for 24 h at various temperatures. After cooling and separation of the H2-clay by filtration, the residue is treated in the same manner as described above. The yields of oxazolidines are given.
The following example is about its application on the synthesis of morphlinopyrimidines [3].
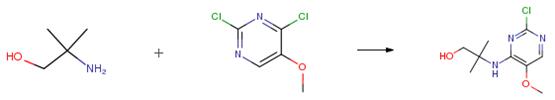
To a stirred solution of 2, 4-dichloro-5-methoxypyrimidine (5 g, 27.93 mmol) in 1, 4-dioxane (50 mL) under argon atmosphere were added diisopropylethylamine (7.2 g,55.86 mmol) and 2-amino-2-methylpropan-1-ol (2.4 g, 27.93 mmol) at RT. The reaction mixture was stirred at 130 °C for 48 h. After consumption of the starting materials (monitored by TLC), the volatile components were evaporated in vacuo. The residue was diluted with a saturated sodium bicarbonate solution (50 mL) and extracted with EtOAc (2 x 50 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo. The crude material was purified by column chromatography using 10percent EtOAc: hexanes to afford 2-((2-chloro-5 -methoxypyrimidin-4-yl) amino)-2-methylpropan- 1 -ol (5.5 g, 84 percent) as a white solid.
The following example is about its application on the synthesis of a vanilloid receptor antagonist [4].
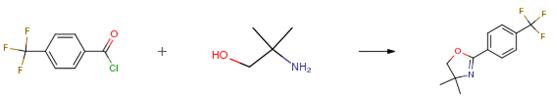
To an ice-cold solution of 2-amino-2-methyl-l-propanol (21.4g, 240mmol) and triethylamine (16.8 mL, 120 mmol) in THF was added dropwise 4- trifluoromethyl benzyl chloride (25.0 g, 120 mmol). After the addition, the reaction mixture was stirred at 00C for 30 mins and then stirred at ambient temperature for 8 hrs. The precipitate was removed by filtration and the filtrate was concentrated by evaporating the solvent under reduced pressure. The resulting residue was treated dropwise with thionyl chloride with vigorous stirring at 00C. After complete addition, the reaction mixture was further stirred at ambient temperature for l hr. Ether was poured into the reaction mixture and the precipitate was collected by filtration. The precipitate was dissolved in water and the aqueous solution was hydrolyzed with 10 percent (w/v) NaOH. The aqueous phase was extracted three times with ether, and the combined organic layer was washed with brine, dried over anhydrous MgSO4, and concentrated under reduced pressure. The crude residue was purified by column chromatography (Hex/EtOAc = 5/1) to yield the product (25.4g, 87percent).
References
1.King Fahd University of Petroleum and Minerals. El AB, Suleiman RK, Ibrahim MB. Solid-supported palladium (ii) complex as a heterogeneous catalyst for cross coupling reactions and methods thereof. US2017/275318[P], 2017, A1.Location in patent: Paragraph 0124-0125
2.Rohand T, Savary J, Markó IE. Synthesis of dioxolanes and oxazolidines by silica gel catalysis[J]. Monatshefte fur Chemie, 2018, 149(8):1429-1436.
3.Forum Pharmaceuticals Inc. Burnett DA, Bursavich MG, Mcriner AJ. Fused morphlinopyrimidines and methods of use thereof WO2015/66696[P], 2015, A1, Location in patent: Paragraph 0389
4.Amorepacific Corporation. Novel compounds, isomer thereof, or pharmaceutically acceptable salts thereof as vanilloid receptor antagonist; and pharmaceutical compositions containing the same. WO2009/96701[P], 2009, A2, Location in patent: Page/Page column 48-49
You may like
Related articles And Qustion
See also
Lastest Price from 2-Amino-2-methyl-1-propanol manufacturers

US $0.00/kg2025-09-24
- CAS:
- 124-68-5
- Min. Order:
- 1kg
- Purity:
- 95%min
- Supply Ability:
- 20tons
US $1.00-4.00/KG2025-09-11
- CAS:
- 124-68-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG