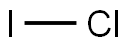Iodine monochloride is a reddish brown liquid or black crystals, there are two variants of crystals. α-type is black needles, stable, ruby red under light. β-type is black flakes, unstable, brownish red under light. It has the odor of chlorine and iodine. It does not absorb moisture but can form iodine pentoxide when contacting air. Soluble in water, ethanol, ether, carbon disulfide, and acetic acid, relative density (d0) α-type 3.1822, (d34) β-type 3.24. Melting point α-type 27.2 ℃, β-type 13.9 ℃. Boiling point α-type 97.4°C, β-type 97.4, 100°C (decomposition). It also exhibits corrosive properties. It has the flavor of chlorine and iodine, and it is oxidative and strongly corrosive, and iodination with organic matter, it also shows chlorination, and it can be used for the substitution of the aromatic ring.

Iodine monochloride is used to estimate theiodine values of fats and oils and as a topicalanti-infective (Merck 1996). For determination of iodine absorption number of fats Iodine monochloride is used as a catalyst in organic synthesis. It is the source of electrophilic iodine in the synthesis of certain aromatic iodides.
Iodine monochloride is prepared by the action of liquid or dry chlorine on astoichiometric quantity of solid iodine. Aqueous solutions of ICl are preparedby passing chlorine gas into a suspension of iodine in moderately stronghydrochloric acid:
5I2 + 4HCl + 3Cl2 → 10ICl + 2H2
Alternatively, iodine monochloride may be made by oxidation of iodine withiodic acid in strong hydrochloric acid solution:
2I2 + HIO3 + 2HCl → 2ICl + 3HIO
Reacts with air to form iodine pentaoxide (I2O5), which decomposes into iodine (I2) and oxygen (O2) with heat beginning at 275°C and proceeding rapidly at 350°C. Soluble in water; reacts with water or steam to produce toxic and corrosive fumes [Lewis].
Iodine monochloride is moderately explosive when heated [Lewis]. Reacts with rubber and many organic materials. Enflames (after a period of delay) with aluminum foil [Mellor 2:119(1946-1947)]. Reacts dangerously with other active metals. Reacts vigorously with cadmium sulfide, lead sulfide, silver sulfide, and zinc sulfide [Mellor 2, Supp. 1:502(1956)]. Combines very exothermically with phosphorus trichloride [Mellor 2, Supp. 1:502(1956)]. Forms Iodine pentaoxide in air which reacts explosively when warmed with carbon, sulfur, sugar, resin, or powdered combustible elements [Mellor 8: 841(1946-1947)].
Toxic by ingestion and inhalation, strong irritant to eyes and skin. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Iodine monochloride is highly corrosive tothe skin. Contact with the skin causes burns and dark patches. Upon contact, washimmediately with 15-20% HCl. Vapors areirritating to the skin, eyes, and mucous mem branes. The compound is moderate to highlytoxic by an oral route. The lethal dose in ratsis 59 mg/kg (NIOSH 1986).
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
reaction type: Redox Reactions
Purify it by repeated fractional crystallisation from its melt at low temperatures. The black crystals melt to a red-brown liquid. [Cornog & Karges Inorg Synth I 165 1939.]