Uses and Hazards of Iodine monochloride
Iodine monochloride is a black crystals or a reddish brown oily liquid with a pungent odor. Melting point 27°C (alpha form) or 14°C (beta form). Corrosive to metals and tissue.

Polarity
Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+.
Uses
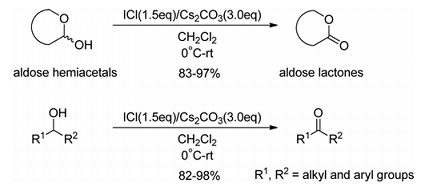
In Wijs' solution (iodine monochloride in glacial acetic acid), used to determine iodine values of fats and oils.
Preparation
Iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation
I2 + Cl2 → 2 ICl
When chlorine gas is passed through iodine crystals, one observes the brown vapor of iodine monochloride. Dark brown iodine monochloride liquid is collected.
Hazards
Iodine Monochloride is a corrosive chemical and contact can severely irritate and burn the skin and eyes with possible eye damage. Breathing Iodine Monochloride can irritate the nose and throat. Breathing Iodine Monochloridecan irritate the lungs causing coughing and/or shortness of breath. Higher exposures can cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency, with severe shortness of breath.