Iodine Monochloride (ICl) as a Highly Efficient
Iodine monochloride (ICl) was discovered to be a highly efficient, green oxidant, which can oxidize aldose hemiacetals, diarylmethanols, arylalkylmethanols, anddialkylmethanols to the corresponding aldose lactones, diarylmethanones, arylalkylmethanones, and dialkylmethanones, respectively, in high yields. ICl as a green, metal-free oxidant is characterized by mild reaction condition, short reaction time, good yield, and broad scope.
INTRODUCTION
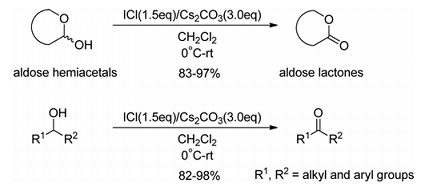
The oxidation of alcohols to the corresponding carbonyl compounds, such as lactones, ketones, and aldehydes, is among the most fundamental, useful, and widely investigated organic reactions. During the study of aryl C-glycosides as SGLT2 inhibitors for the treatment of type 2 diabetes in our laboratories,[1,2] a variety of glyconolactones as the glycosyl donors were needed during the synthesis. Glyconolactones 1, in particular gluconolactones, are often used as the glycosyl donors for the synthesis of biologically active C-glycosides in carbohydrate chemistry and medicinal chemistry,[3–5]which are usually synthesized by oxidation of the corresponding aldose hemiacetals 2 with a variety of oxidants[6–18] (Scheme 1). During the synthesis of glyconolactones on pilot scales in our laboratories, a green, metal-free oxidant that is characterized by being cheap, mild reaction condition, short reaction time, and good yield was urgently needed.
However, among the oxidants currently used for the oxidation of aldose hemiacetals to lactones aforementioned, I2=K2CO3 is still the most promising one because it is only associated with prolonged reaction time and a large quantity of oxidant (3 eq), which are unacceptable in large-scale synthesis,[17] but does not suffer from other serious drawbacks aforementioned for the other oxidants. This fact prompted us to study the mechanism of this oxidant with anticipation that based on its mechanism we could find a new oxidant that could meet our criteria. As shown in Scheme 2, the oxidation action of I2=K2CO3 relies on the disproportionation of I2 in the presence of a base to produce an iodonium species, which is in turn bound to the O atom of OH in an alcohol to be oxidized, followed by elimination of HI to complete the oxidation.[17,19] According to this mechanism, we proposed ICl as the more powerful analog of I2 for this purpose. ICl is widely used in inorganic and organometallic chemistry[20,21] and sometimes is an iodinating reagent for aromatics[22,23] and alkynes[24] in synthetic organic chemistry. However, it has never been used for the oxidation of alcohols to corresponding carbonyl compounds to the best of our knowledge. ICl should be capable of producing iodonium species much more readily and faster than I2 due to the larger polarization of the I-Cl bond than the I-I bond in I2 (Scheme 2). We herein report the systematic study of ICl as the oxidant to oxidize alcohols to the corresponding carbonyl compounds, including the optimization of the reaction conditions and the exploration of the scope of alcohol substrates.
RESULTS AND DISCUSSION
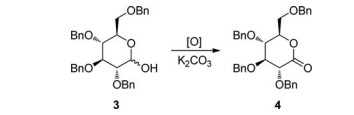
Because ICl was proposed to replace I2 to achieve the oxidation with anticipation of shorter reaction time and less quantity of oxidant, comparative experiments with both of them were then conducted in the preliminary attempts (Table 1). 2,3,4,6- Tetra-O-benzyl-D-glucopyranose 3 was selected as the aldose hemiacetal substrate in both the preliminary attempts and the optimization of reaction conditions (Tables 1 and 2) because it was readily available and crystalline and the corresponding product 2,3,4,6-tetra-O-benzyl-D-gluconolactone 4 was commonly used in the synthesis of C-glucosides[25–27] (Table 1).
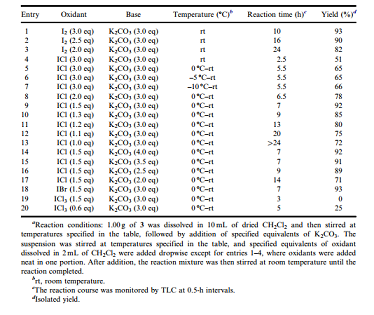
After the proposal of ICl as an oxidant for the oxidation of aldose hemiacetals to lactones, we designed a number of parallel experiments to preliminarily test the feasibility of this oxidant under the reaction condition initially used for I2 [17] because both of them were believed to act through an almost identical mechanism. As shown in entries 1–3 in Table 1, with 3.0 eq of K2CO3 as base, 2.0–3.0 eq of I2 were tested (added neat to the reaction mixture in one portion), and it turned out that as the quantity of I2 decreased, the reaction time increased dramatically and the isolated yields of 4 decreased significantly, indicating that 3.0 eq of I2 was really necessary to ensure a short reaction time and a good yield.
In conclusion, we have discovered a highly efficient, green oxidant, iodine monochloride (ICl), and optimized the reaction conditions, including the equivalent of ICl, the base and its equivalent, reaction temperature, and solvent. Compared to the I2=K2CO3 system, the ICl=Cs2CO3 system is characterized by less quantity of oxidant (1.5 eq of ICl vs. 3 eq of I2), shorter reaction time, and greater yield. The substrate scope of the oxidant has been intensively studied, and it is applicable to a variety of alcohol substrates with short reaction times and good yields, such as aldose hemicacetals, diarylmethanols, arylalkylmethanols, and dialkylmethanols. However, it is not applicable to olefin-bearing and primary alcohols.
EXPERIMENTAL
Melting points were determined with an X-4 microscopic melting-point apparatus and are uncorrected. Infrared (IR) spectrum was recorded on a Bruker Vector 22 FT-IR spectrometer as thin films. 1 H NMR and 13C NMR spectra were taken on a Bruker AV400 nuclear magnetic resonance spectrometer, using DMSO-d6 or CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. High-resolution mass spectra (HR-MS) were obtained with an Agilent Q-TOF 6510 mass spectrometer with electro-spray ionization (ESI) technique and direct injection method.
Starting aldose hemiacetals 3, 5, 7, 9, 11, 13, 15, and 17 were prepared according to known procedures,[1,31–34] and other starting alcohols 19, 21, 23, 25, 27, 29, 31, 33, 35, 37, 39, 41, and 43 were commercially available.