Manufacture of dry laundry bleaches,
detergent-bleach washing compound, scouring
powders, plastic dishware cleaners, and metal
cleaners; hair-wave neutralizers, pharmaceuticals;
general oxidizing reactions.
Colorless, beautiful crystals; hygroscopic; pure material stable
for a few days, although with slight loss of active oxygen, the rate
of decomposition is catalysed by the impurities formed; vigorous
oxidizing agent. M.p. +45°C with slight decomposition.
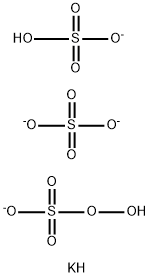
Potassium peroxosulfate is used for bleaching polyamide and cellulose fibers. However, it is ordinarily used only to clean wool and to reduce its shrinkage.
This is a stable form of Caro's acid and should contain >4.7% of active oxygen. It can be used in EtOH/H2O and EtOH/AcOH/H2O solutions. If active oxygen is too low. it is best to prepare it afresh from 1mole of KHSO5, 0.5mole of KHSO4 and 0.5mole of K2SO4. [Kennedy & Stock J Org Chem 25 1901 1960, Stephenson US Patent 2,802,722 1957.] A rapid preparation of Caro's acid is made by stirring finely powdered potassium persulfate (M 270.3) into ice-cold conc H2SO4 (7mL) and when homogeneous add ice (40-50g). It is stable for several days if kept cold. Keep away from organic matter as it is a STRONG OXIDANT. A detailed preparation of Caro's acid (hypersulfuric acid, H2SO5) in crystalline form m ~45o from H2O2 and chlorosulfonic acid was described by Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 388 1963.