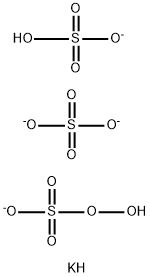
Potassium peroxymonosulfate, an Efficient Disinfectant Agent
Basic Introduction
Potassium Sulfate is a moderately water and acid soluble Potassium source for uses compatible with sulfates. [1]Sulfate compounds are salts or esters of sulfuric acid formed by replacing one or both of the hydrogens with a metal. Most metal sulfate compounds are readily soluble in water for uses such as water treatment, unlike fluorides and oxides which tend to be insoluble. Organometallic forms are soluble in organic solutions and sometimes in both aqueous and organic solutions. Metallic ions can also be dispersed utilizing suspended or coated nanoparticles and deposited utilizing sputtering targets and evaporation materials for uses such as solar cells and fuel cells. Potassium Sulfate is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. We also produce Potassium Sulfate Solution. American Elements produces to many standard grades when applicable, including Mil Spec (military grade); ACS, Reagent and Technical Grade; Food, Agricultural and Pharmaceutical Grade; Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follows applicable ASTM testing standards. Typical and custom packaging is available. Additional technical, research and safety (MSDS) information is available as is a Reference Calculator for converting relevant units of measurement. Technical guidance for using Potassium Sulfate in agriculture is also available.
The Fundamental Properties of Elemental Constituents
Potassium (atomic symbol: K, atomic number: 19) is a Block S, Group 1, Period 4 element with an atomic weight of 39.0983. The number of electrons in each of Potassium's shells is [2, 8, 8, 1] and its electron configuration is [Ar] 4s1. The potassium atom has a radius of 227.2 pm and a Van der Waals radius of 275 pm. Potassium was discovered and first isolated by Sir Humphrey Davy in 1807. Potassium is the seventh most abundant element on earth. It is one of the most reactive and electropositive of all metals and rapidly oxidizes. As with other alkali metals, potassium decomposes in water with the evolution of hydrogen because of its reacts violently with water, it only occurs in nature in ionic salts. In its elemental form, potassium has a silvery gray metallic appearance, but its compounds (such as potassium hydroxide) are more frequently used in industrial and chemical applications. The origin of the element's name comes from the English word 'potash,' meaning pot ashes, and the Arabic word qali, which means alkali. The symbol K originates from the Latin word kalium. Sulfur (or Sulphur) (atomic symbol: S, atomic number: 16) is a Block P, Group 16, Period 3 element with an atomic radius of 32.066. The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.

Potassium peroxymonosulfate as an Effective Sanitizing Agent
Researchers suggest that Potassium peroxymonosulfate could be used as an alternative disinfectant for biosecurity enhancement in animal farms or hospitals. Potassium peroxymonosulfate was evaluated for bactericidal and virucidal effects against Salmonella Infantis(SI)Escherichia coli,rifampicin-resistant Salmonella Infantis(SI-rif), Newcastle disease virus (NDV),and avian influenza virus (AlV),in the absence or presence of organic materials. In addition,inactivation activity toward a virus on virus-spiked clothes was also examined. Potassium monopersulfate could inactivate SI,E.coli, and Sl-rif even in the presence of organic materials under various concentrations and exposure/contact time conditions. PMPS couldalso inactivate NDV and AlV. In addition, Potassium peroxymonosulfate could inactpivate AlV on a virus-spiked rayon sheet. In conclusion, the present study showed that Potassium peroxymonosulfate has good antimicrobial properties against Sl,E.coli, Sl-rif,NDV, and AlV when used at the optimal dosage and exposure timing.[2]
Generally, foods derived from animal products, such as eggs, meat, and milk, have been implicated as vehicles of one or more of pathogens causing food-borne illness, especially Escherichia coli and Salmonella spp. In Japan, hazard analysis and critical control points (HACCP) have been introduced at animal farms for food safety. One of the key points for HACCP is the appropriate usage of disinfectants to enhance biosecurity on and around animal farms.Normally, poultry virus diseases such as Newcastle disease (ND) and avian influenza (AI), have a strong negative economic impact on the poultry industry. The viruses of both diseases are excreted in large amounts from the respiratory and digestive systems of clinically infected birds and contaminate the environment. Hence, an important aspect of disease control consists of proper cleaning and disinfection of the farm environment, which depends upon the use of an effective disinfectant agent. Many disinfectants are commercially available, and it is important to ensure that the disinfectant being used is effective against various pathogens.
The appropriate usage of disinfectants is critical for establishing a successful sanitation program. Because not all disinfectants are effective against major pathogens, different families of disinfectants that target specific microorganisms should be considered. For instance, several bacteria and viruses are sensitive to phenols; however, most bacteria are also sensitive to quaternary ammonium, iodophors, paracetic acid, glutaraldehydes, and cresols. Therefore, there is no single disinfectant reported in the literature that would be efficacious against a wide spectrum of etiological agents that economically impact diseases in animal farms. Moreover, special care should be taken when applying the disinfectant as it should be safe for both animals and humans. In addition, the hardness of water, correct dilutions, duration of contact with the pathogens, and the presence of organic material should also be taken into consideration.The aim of the present study was to evaluate efficacies of potassium monopersulfate (PMPS) against Salmonella Infantis (SI), E. coli, rifampicin-resistant Salmonella Infantis (Sl-rif), Newcastle disease virus (NDV), and avian influenza virus (AIV), in the absence or presence of organic materials, as well as to evaluate the inactivation activity of PMPS toward AIV on a rayon sheet for biosecurity applications in animal farms and animal hospitals.[3]
Potassium peroxymonosulfate is the potassium salt of peroxymonosulfuric acid, which is widely used as an oxidizing agent. This salt probably acts on bacteria by oxidation. It also attacks viral protein capsids, thereby releasing and inactivating the nucleic acids of viruses, thus affecting the bactericidal and virucidal efficacies under various concentrations, exposure/contact times, and organic material conditions. The results also indicated that bacteria were more sensitive than viruses to inactivation by Potassium peroxymonosulfate. Several products, such as Oxone and DupontTM, contain potassium monopersulfate for their main ingredient, as a non-chlorine shock agent; PMPS breaks the chorine-ammonia bond formed when chlorine combines with ammonia, without increasing the chlorine level of the swimming pool; hence, PMPS can be used in swimming pools to keep the water clear. In addition, Virkon-S contains PMPS at 21.41% and has been used at concentrations of 5,000-20,000 ppm for multipurpose virucidal disinfection, with the greatest numbers of the Environmental Protection Agency (EPA Registration No. 71654-6)-registered claims, against pathogens affecting domestic and companion animals. However, the Potassium peroxymonosulfate tablet in the present study was used at concentrations of 312.5-5,000 ppm; hence, this PMPS is confirmed for safety and toxicity towards animals and humans. Generally, bacteria and viruses are highly resistant to disinfectants contained in bio-environmental constituents such as feces, saliva, or vomitus. In addition, viruses can survive on the surfaces of materials, fomites, and food for long periods. Our model of evaluating virus inactivation involved utilizing rayon sheets to simulate carpets, bedding, towels, or cloths contaminated with viruses and containing organic materials. In addition, several quaternary ammonium compounds have been reported to exhibit broad-spectrum virucidal activity, including in the present study that used rayon; therefore, considering the cloth savings and its efficacy, DTAB was selected for comparison with PMPS. As shown in Table 7, 5,000, 2,500 and 1,250 ppm of PMPS could inactivate AIV, while DTAB could not inactivate this virus on rayon sheets at the recommended dose. These results suggest that PMPS can be applied as a disinfectant or a virucidal agent that can inactivate AIV in contaminated carpets, clothes, towels, or bedding, especially in animal farms or hospitals.
References:
[1] Ravi K Y ,Kavitha S ,Pugazhendi A , et al.Effect of potassium persulphate-induced microwave pretreatment for cost-effective sludge solubilization and bioenergy recovery[J].Chemical Engineering Journal,2024,498155111-155111.
[2]YuYing H ,Juan M ,EnShuai D , et al.Effect and its mechanism of potassium persulfate on aerobic composting process of vegetable wastes.[J].Environmental science and pollution research international,2023,31(5):7111-7121.
[3]Haifu Z ,Xinhuan L ,Xixi L , et al.Potassium Persulfate Initiated Air Epoxidation of Olefins over Co-MOF Efficiently[J].Russian Journal of Physical Chemistry A,2022,96(9):1925-1934.
Related articles And Qustion
Lastest Price from POTASSIUM PEROXYMONOSULFATE manufacturers

US $10.00/KG2025-04-21
- CAS:
- 37222-66-5
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 37222-66-5
- Min. Order:
- 1kg
- Purity:
- 42.5%
- Supply Ability:
- 20MT