protein tyrosine kinase
Protein tyrosine kinase inhibitor PTK is a group of enzymes, which catalyze the phosphate group in the ATP to be transferred to the many important tyrosine residues in the protein, resulting in residues phosphorylation, thereby activating various substrates enzymes, affecting the growth, proliferation and differentiation of cells through a series of reactions. So far, it has been proved that many PTK inhibitors have anti-cancer activity, and some can induce differentiation of leukemia cells; the combination of PTK inhibitors with other anti-cancer drugs in the treatment of cancer has also achieved some encouraging results.
(1) Receptor tyrosine kinase (RTK) is a large and important class of cell surface receptor family. All RTK is composed of three parts:
(1) containing the extracellular domain of the ligand binding site,
(2) single-transmembrane hydrophobic α helix region,
(3) intracellular domain containing the tyrosine kinase activity. It has been found of more than 50 different RTK and can be divided into six subfamilies according to the structure (Figure 32-15). Its extracellular ligand is a soluble or membrane bound polypeptide or protein hormones, including insulin and various growth factors, mainly including epidermal growth factor (EGF), platelet-derived growth factor (PDGF), macrophage colony stimulating factor ( M-CSF), insulin, insulin-like growth factor -1 (IGF-1), hepatocyte growth factor (HGF), nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) and so on.
(2) The activation of the receptor tyrosine kinase is a very complex process. First it need to have the ligand-mediated dimerization, leading to the formation of homologous or heterogonous dimer through dimerization, thus achieving the phosphorylation of the tyrosine of the intracellular region of the receptor of each other inside the dimer, namely, the achievement of auto-phosphorylation of the receptor. It has been now found, the ligand-mediated receptor dimerization (oligomerization) is the universal mechanism of activating single-transmembrane enzymes coupled receptor. For different types of ligands, the specific mechanism may be slightly different: some ligands are dimer themselves (e.g., PDGF, etc.), which contain the surface of the two binding receptors, allowing the cross-link of the two receptors together; some ligand is monomer type (e.g. hGH), but their surfaces have two different sites that can be contacted with two receptor molecules to form a 1: 2 ratio of ligand - receptor complex. Furthermore, fibroblast growth factor itself is a monomer, and can’t induce the dimerization of the receptor, requiring forming multivalent complex with heparin sulfate proteoglycan, thereby combining the two or more receptors.
(3) Imatinib mesylate belongs to a new type of tyrosine kinase inhibitor, belonging to the derivatives of 2-phenylaminopyrimidine. About 95% of chronic myelogenous leukemia (CML) patients had Ph1 chromosome-positive, namely the proto-oncogene ABL in the chromosome 9 undergoes ectopia into the part of the chromosome 22 called the breakpoint cluster region (BCR) that is a oncogene; the two kinds of genes get recombination together, producing a fusion protein p-210, compared with the normal C-ABL protein p-150, p-210 has high tyrosine kinase activity, being able to stimulate the proliferation of white blood cells, leading to leukemia. The drug can strongly inhibit the activity of ABL tyrosine kinase activity both in vivo and in vitro, specifically inhibiting the expression of ABL as well as the proliferation of the BCR-ABL cells, thereby can be used for the treatment of CML. In addition, the drug is capable of inhibiting the tyrosine kinase of the platelet-derived growth factor (PDGF) and stem cell factor (SCF) receptor, and can inhibit the PDGF and SCF-mediated biochemical reactions, but does not affect the signaling transduction of other stimulating factors such as epidermal growth factor.
(4) Tyrosine kinase receptor pathways: tyrosine kinase receptor pathway is one of the most important networks in the intracellular signaling pathways. The signals of most growth factors exert the regulatory function through this signaling pathway. Growth factor receptors have tyrosine kinase activity themselves, or alternatively play a role through binding to intra-membrane tyrosine protein kinases. Protein tyrosine kinases consists of four main components: the part located outside the cell is the ligand recognition and binding site, followed by a part of transmembrane structure and the intracellular part is the catalytic region of the tyrosine protein kinase; the adjustment portion is located at the carboxyl terminus of the peptide tail. When the agonist binds to the extracellular membrane recognition site, the intra-membrane tyrosine protein kinase will be activated, subject to auto-phosphorylation, and then change the action of the effector. Various kinds of structural changes with some of them being the mutations in a very small number of amino acid residues of the key region can lead to emergence of the oncogene of tyrosine kinase class which has been loss of control.
(5) Receptor tyrosine protein kinase: tyrosine protein kinase (TPK) exerts its function through making the tyrosine residues of the substrate protein subject to phosphorylation. It includes the receptor type and non-receptor type.
This class of receptor has the protein tyrosine kinase activity itself with their ligands being mostly multi-cell growth factor such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (EGF) and fibroblast growth factor (FGF) and so on. After the binding of ligand to the receptor, through itself and the phosphorylation of the tyrosine residues of the substrate protein and thus triggered enzymatic cascades, the ligand-receptor can regulate cell growth, differentiation and metabolism.
Such receptor molecules can be divided into three structural regions, namely, the extracellular ligand binding domain, intracellular tyrosine kinase active region as well as the transmembrane regions connecting the above two structure domains. Such receptor not only has tyrosine protein kinase activity but also contains the phosphorylation site of tyrosine residue itself, meaning that it can be subject to tyrosine phosphorylation itself. When the ligand binds to the receptor, the receptor can undergo dimerization, activating the tyrosine kinase activity of the receptor as well as making their tyrosine residues subject to phosphorylation. Moreover, the tyrosine auto-phosphorylation can cause its conformational change and make it become the recognition sites of the tyrosine kinase substrates to be recognized by downstream signaling molecules, thus promoting the signal transmission and amplification through cascade phosphorylation reactions.
(6) Receptor of the protein tyrosine kinases: This class of receptor doesn’t have tyrosine kinase activity itself, but its cytoplasmic region contains the tyrosine kinase activity site, when the receptor binds with the ligand, being able to bind to and activate the JAK protein-tyrosine kinases, thus initiating the intracellular signal transduction.
- Structure:
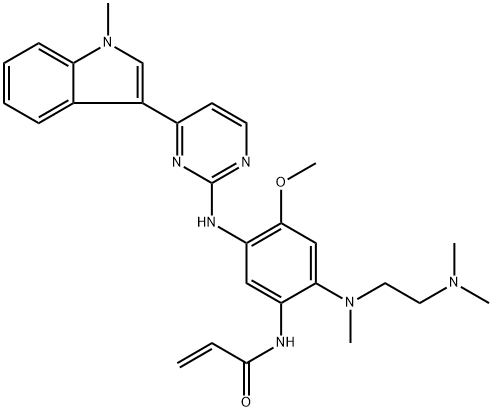
- Chemical Name:AZD-9291
- CAS:1421373-65-0
- MF:C28H33N7O2
- Structure:
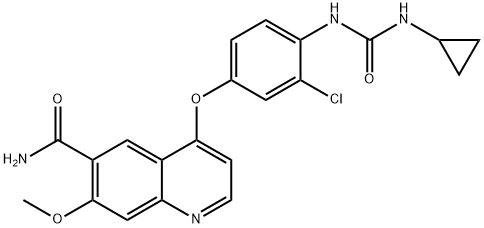
- Chemical Name:Lenvatinib
- CAS:417716-92-8
- MF:C21H19ClN4O4
- Structure:
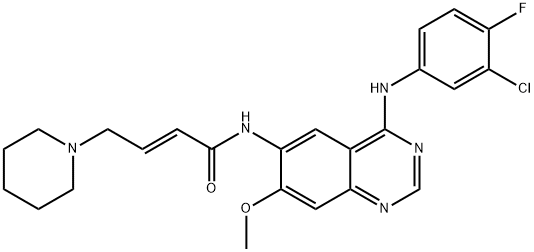
- Chemical Name:Dacomitinib (PF299804)
- CAS:1110813-31-4
- MF:C24H25ClFN5O2
- Structure:
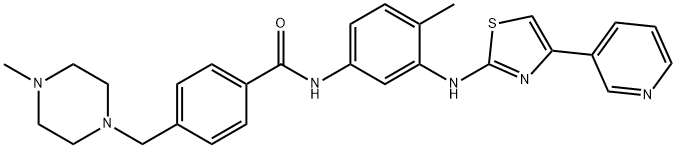
- Chemical Name:Masitinib
- CAS:790299-79-5
- MF:C28H30N6OS
- Structure:
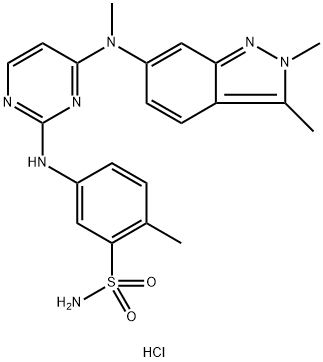
- Chemical Name:Pazopanib Hydrochloride
- CAS:635702-64-6
- MF:C21H24ClN7O2S
- Structure:
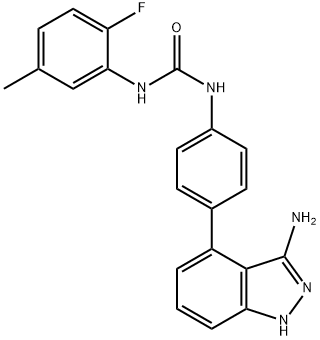
- Chemical Name:Linifanib (ABT-869)
- CAS:796967-16-3
- MF:C21H18FN5O
- Structure:
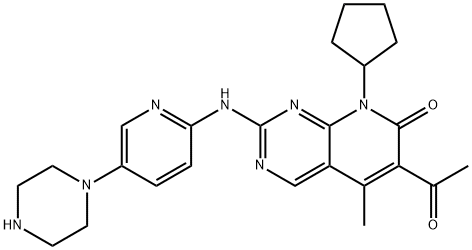
- Chemical Name:Palbociclib
- CAS:571190-30-2
- MF:C24H29N7O2
- Structure:
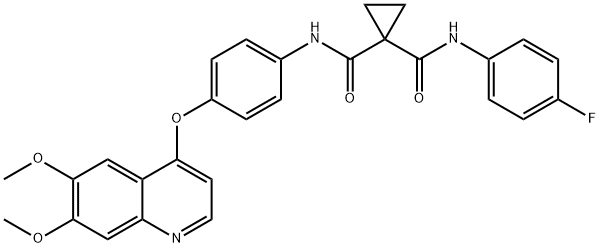
- Chemical Name:Cabozantinib
- CAS:849217-68-1
- MF:C28H24FN3O5
- Structure:
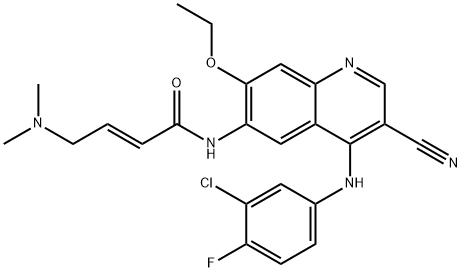
- Chemical Name:PELITINIB
- CAS:257933-82-7
- MF:C24H23ClFN5O2
- Structure:
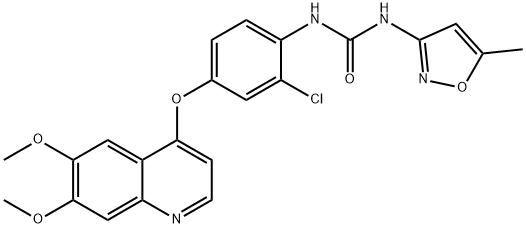
- Chemical Name:Tivozanib
- CAS:475108-18-0
- MF:C22H19ClN4O5
- Structure:
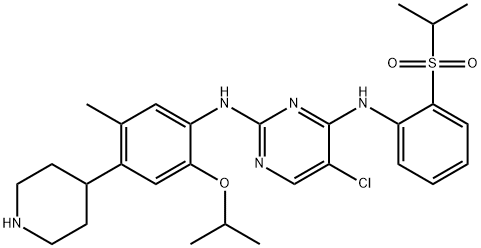
- Chemical Name:Ceritinib (LDK378)
- CAS:1032900-25-6
- MF:C28H36ClN5O3S
- Structure:
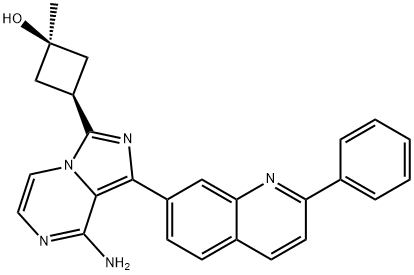
- Chemical Name:Linsitinib
- CAS:867160-71-2
- MF:C26H23N5O
- Structure:
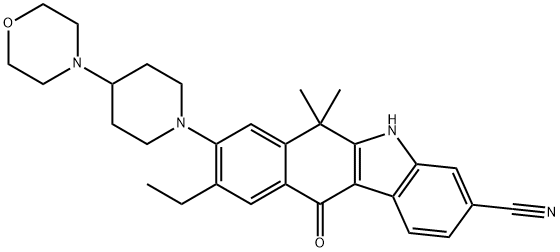
- Chemical Name:Alectinib
- CAS:1256580-46-7
- MF:C30H34N4O2
- Structure:
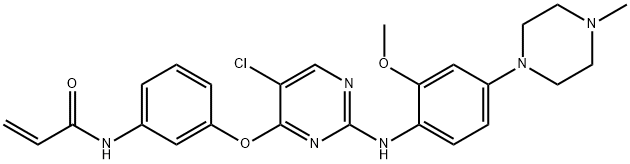
- Chemical Name:WZ4002
- CAS:1213269-23-8
- MF:C25H27ClN6O3
- Structure:
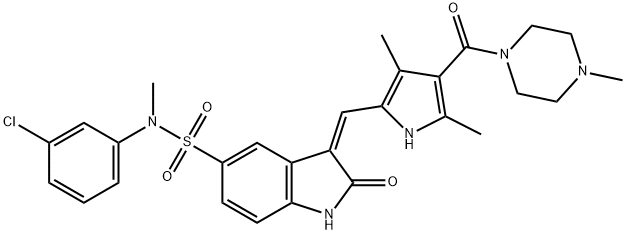
- Chemical Name:SU 11274
- CAS:658084-23-2
- MF:C28H30ClN5O4S
- Structure:
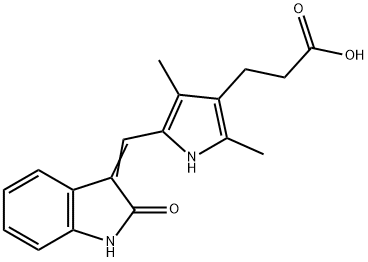
- Chemical Name:Orantinib (SU6668)
- CAS:252916-29-3
- MF:C18H18N2O3
- Structure:
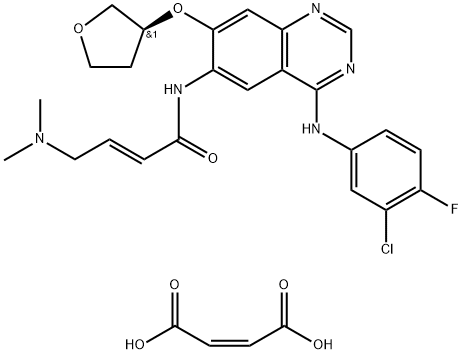
- Chemical Name:Afatinib dimaleate
- CAS:850140-73-7
- MF:C28H29ClFN5O7
- Structure:
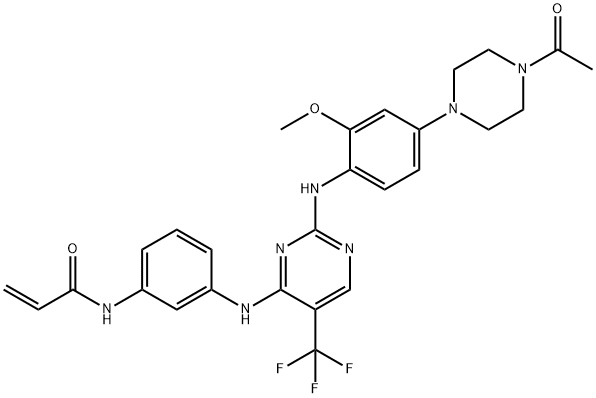
- Chemical Name:CO-1686
- CAS:1374640-70-6
- MF:C27H28F3N7O3
- Structure:
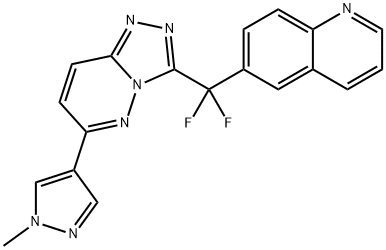
- Chemical Name:J&J Ex-61
- CAS:943540-75-8
- MF:C19H13F2N7
- Structure:
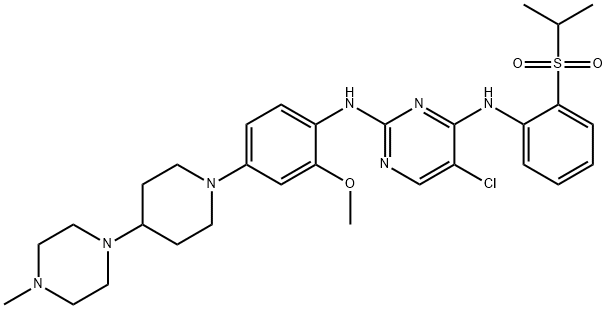
- Chemical Name:NVP-TAE684
- CAS:761439-42-3
- MF:C30H40ClN7O3S
- Structure:
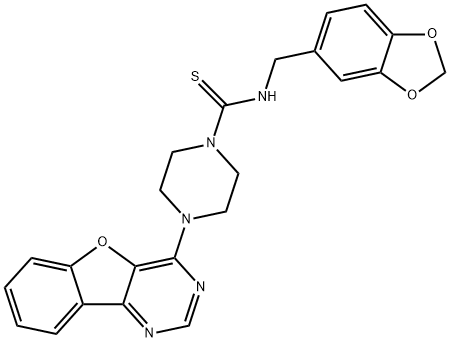
- Chemical Name:Amuvatinib
- CAS:850879-09-3
- MF:C23H21N5O3S
- Structure:
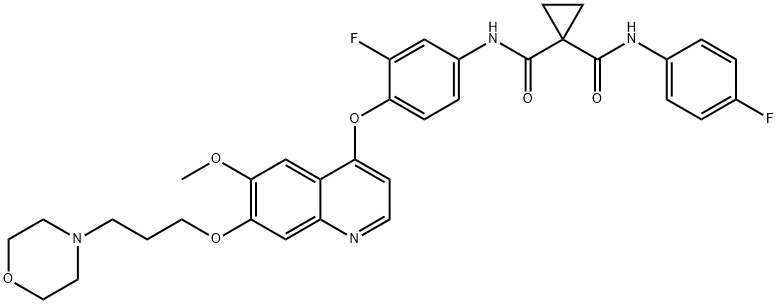
- Chemical Name:Foretinib (GSK1363089)
- CAS:849217-64-7
- MF:C34H34F2N4O6
- Structure:
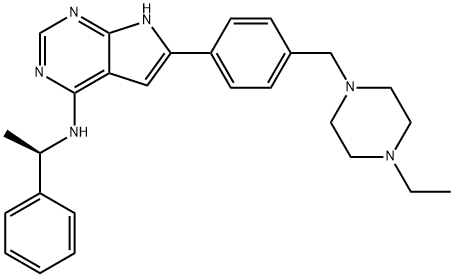
- Chemical Name:AEE788
- CAS:497839-62-0
- MF:C27H32N6
- Structure:
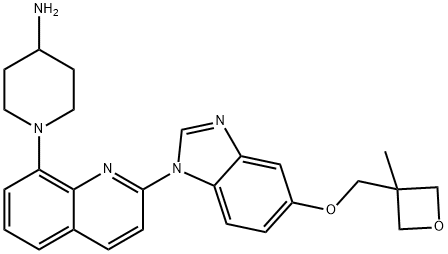
- Chemical Name:Crenolanib
- CAS:670220-88-9
- MF:C26H29N5O2
- Structure:
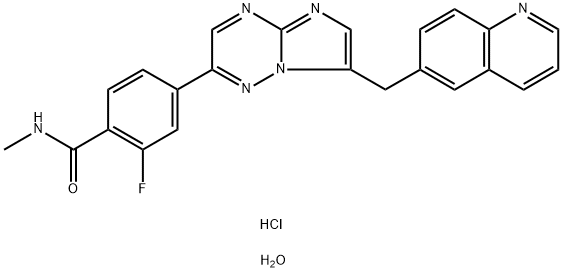
- Chemical Name:Capmatinib hydrochloride
- CAS:1865733-40-9
- MF:C23H20ClFN6O2
- Structure:
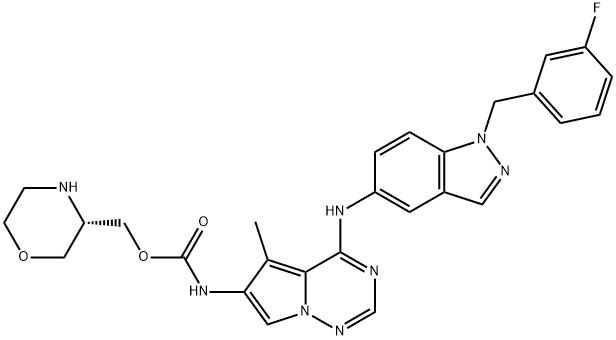
- Chemical Name:AC480 (BMS-599626)
- CAS:714971-09-2
- MF:C27H27FN8O3
- Structure:
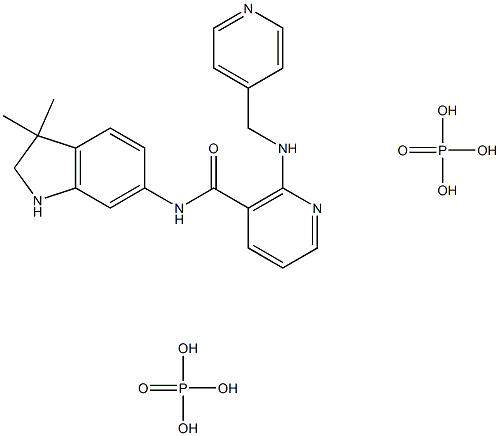
- Chemical Name:AMG-706
- CAS:857876-30-3
- MF:C22H29N5O9P2
- Structure:
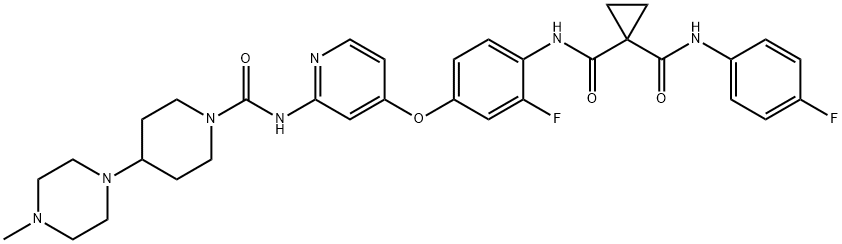
- Chemical Name:Golvatinib (E7050)
- CAS:928037-13-2
- MF:C33H37F2N7O4
- Structure:
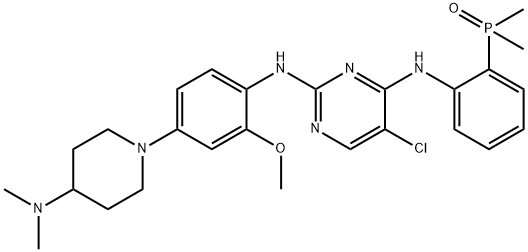
- Chemical Name:AP26113
- CAS:1197958-12-5
- MF:C26H34ClN6O2P
- Structure:
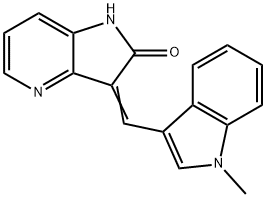
- Chemical Name:GW441756
- CAS:504433-23-2
- MF:C17H13N3O
- Structure:
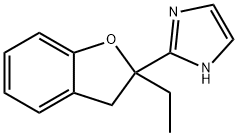
- Chemical Name:2-(2-ETHYL-2,3-DIHYDRO-2-BENZOFURANYL)-1H-IMIDAZOLE
- CAS:189224-48-4
- MF:C13H14N2O
- Structure:
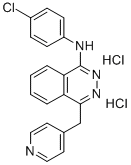
- Chemical Name:Vatalanib Dihydrochloride
- CAS:212141-51-0
- MF:C20H17Cl3N4
- Structure:
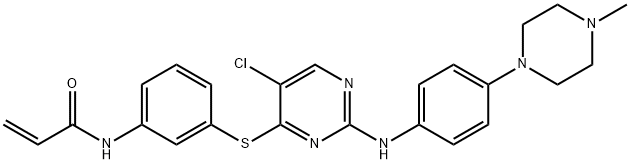
- Chemical Name:WZ8040
- CAS:1214265-57-2
- MF:C24H25ClN6OS
- Structure:
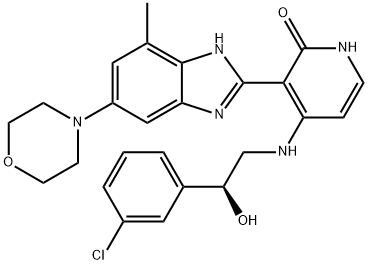
- Chemical Name:Insulin-like Growth Factor-1 Receptor Inhibitor
- CAS:468740-43-4
- MF:C25H26ClN5O3
- Structure:
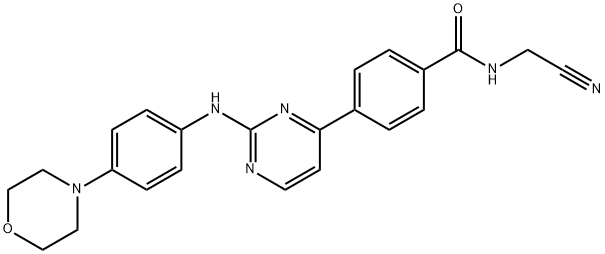
- Chemical Name:Momelotinib
- CAS:1056634-68-4
- MF:C23H22N6O2
- Structure:
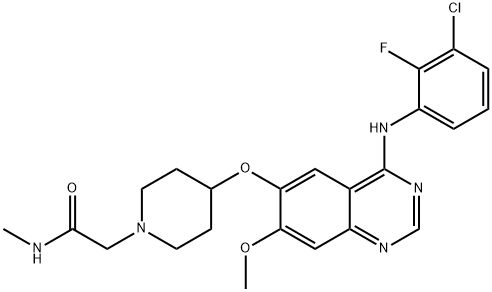
- Chemical Name:AZD8931
- CAS:848942-61-0
- MF:C23H25ClFN5O3
- Structure:
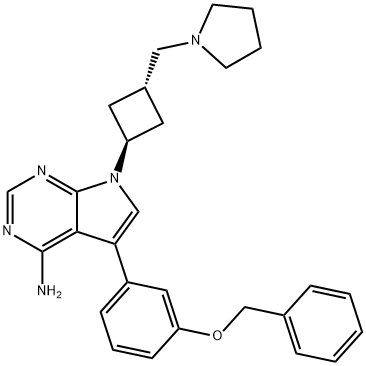
- Chemical Name:5-(3-Benzyloxyphenyl)-7-[trans-3-[(pyrrolidin-1-yl)methyl]cyclobutyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine
- CAS:475488-23-4
- MF:C28H31N5O
- Structure:
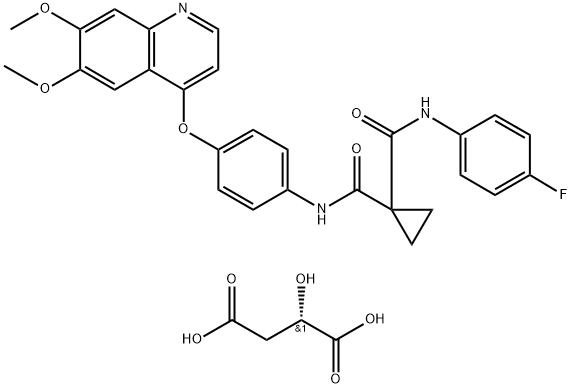
- Chemical Name:Cabozantinib Malate
- CAS:1140909-48-3
- MF:C32H30FN3O10
- Structure:
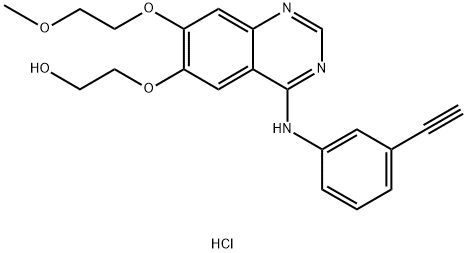
- Chemical Name:OSI-420
- CAS:183320-51-6
- MF:C21H22ClN3O4
- Structure:
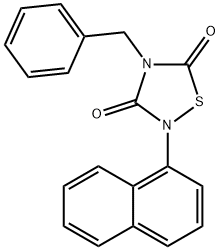
- Chemical Name:4-Benzyl-2-(naphthalen-1-yl)-[1,2,4]thiadiazolidine-3,5-dione
- CAS:865854-05-3
- MF:C19H14N2O2S
- Structure:
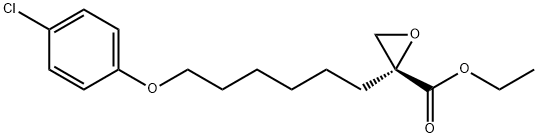
- Chemical Name:R-(+)-Etomoxir
- CAS:124083-20-1
- MF:C17H23ClO4
- Structure:
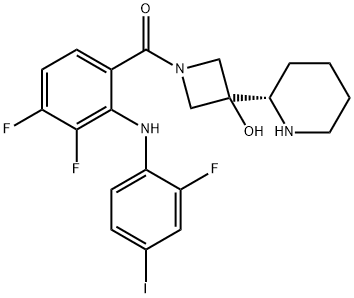
- Chemical Name:XL518
- CAS:934660-93-2
- MF:C21H21F3IN3O2
- Structure:
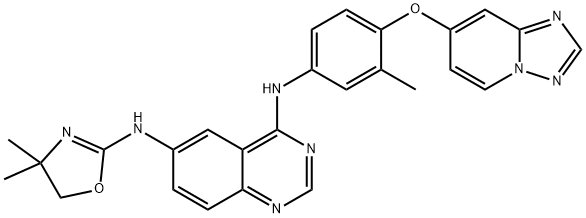
- Chemical Name:Tucatinib
- CAS:937263-43-9
- MF:C26H24N8O2
- Structure:
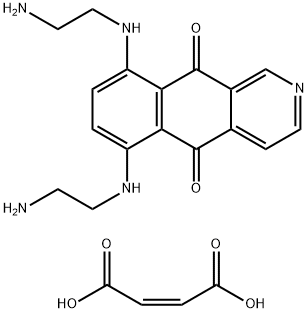
- Chemical Name:Pixantrone Dimaleate
- CAS:144675-97-8
- MF:C21H23N5O6
- Structure:
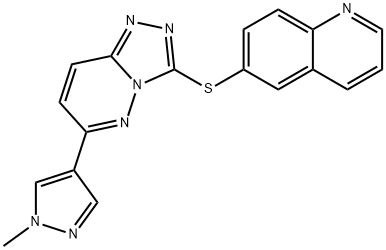
- Chemical Name:SGX-523
- CAS:1022150-57-7
- MF:C18H13N7S
- Structure:
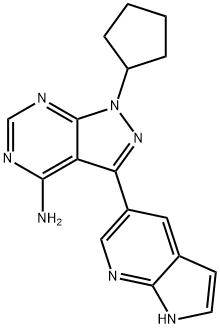
- Chemical Name:1-Cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- CAS:1092788-83-4
- MF:C17H17N7
- Structure:
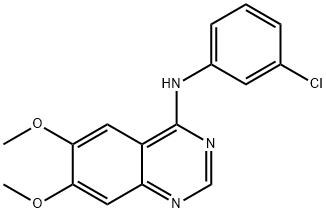
- Chemical Name:AG 1478 HYDROCHLORIDE
- CAS:153436-53-4
- MF:C16H14ClN3O2
- Structure:
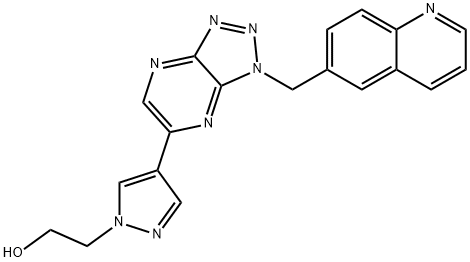
- Chemical Name:PD04217903
- CAS:956905-27-4
- MF:C19H16N8O
- Structure:
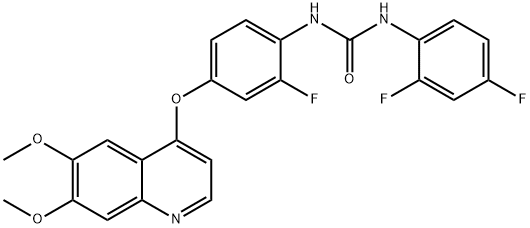
- Chemical Name:Ki8751
- CAS:228559-41-9
- MF:C24H18F3N3O4
- Structure:
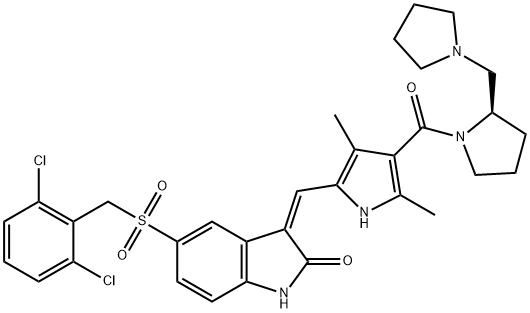
- Chemical Name:(2R)-1-[[5-[(Z)-[5-[[(2,6-DICHLOROPHENYL)METHYL]SULFONYL]-1,2-DIHYDRO-2-OXO-3H-INDOL-3-YLIDENE]METHYL]-2,4-DIMETHYL-1H-PYRROL-3-YL]CARBONYL]-2-(1-PYRROLIDINYLMETHYL)PYRROLIDINE
- CAS:477575-56-7
- MF:C32H34Cl2N4O4S
- Structure:
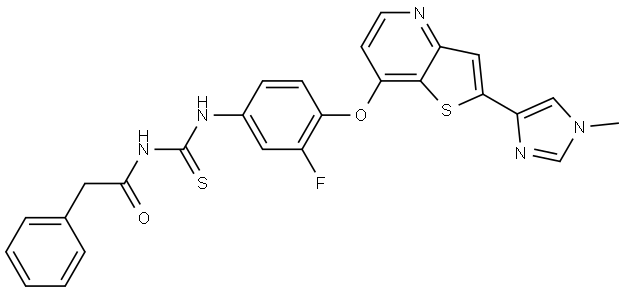
- Chemical Name:N-(3-fluoro-4-(2-(1-methyl-1H-imidazol-4-yl)thieno[3,2-b]pyridin-7-yloxy)phenylcarbamothioyl)-2-phenylacetamide
- CAS:875337-44-3
- MF:C26H20FN5O2S2
- Structure:
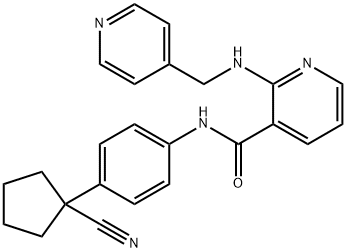
- Chemical Name:Apatinib
- CAS:811803-05-1
- MF:C24H23N5O
- Structure:
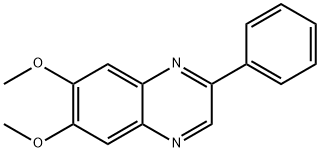
- Chemical Name:TYRPHOSTIN AG 1296
- CAS:146535-11-7
- MF:C16H14N2O2
- Structure:
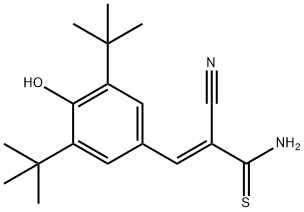
- Chemical Name:TYRPHOSTIN AG 879
- CAS:148741-30-4
- MF:C18H24N2OS
- Structure:
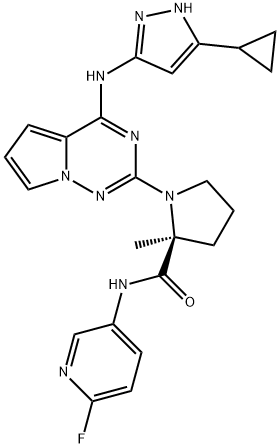
- Chemical Name:BMS-754807
- CAS:1001350-96-4
- MF:C23H24FN9O
- Structure:
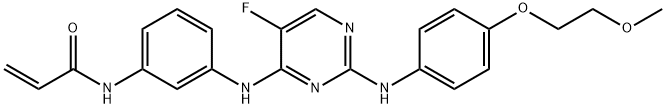
- Chemical Name:AVL-292
- CAS:1202757-89-8
- MF:C22H22FN5O3
- Structure:
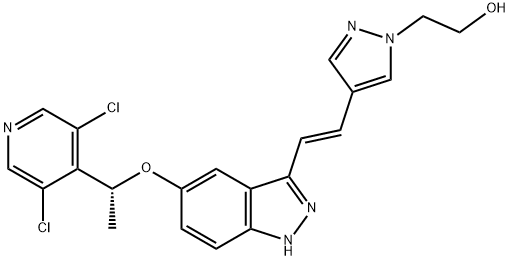
- Chemical Name:LY 2874455
- CAS:1254473-64-7
- MF:C21H19Cl2N5O2
- Structure:
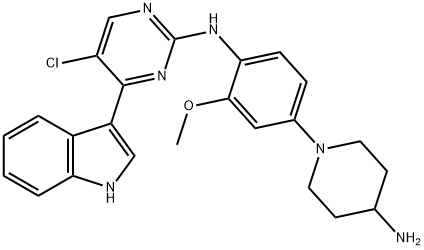
- Chemical Name:AVP-AEW541
- CAS:475489-16-8
- MF:C27H29N5O
- Structure:
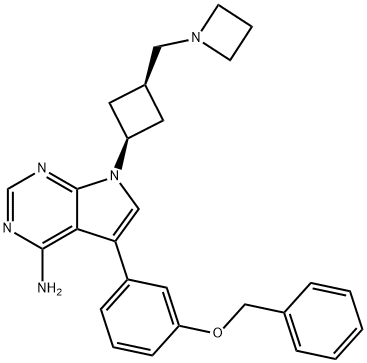
- Chemical Name:ALK/IGF1R inhibitor
- CAS:1356962-20-3
- MF:C24H25ClN6O
- Structure:
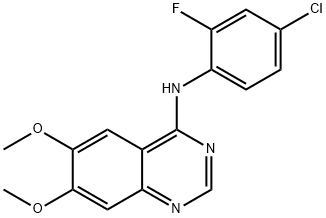
- Chemical Name:ZM 306416
- CAS:690206-97-4
- MF:C16H13ClFN3O2
- Structure:
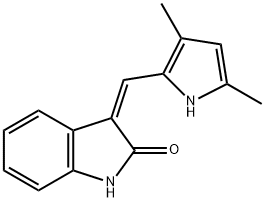
- Chemical Name:3-[(2,4-Dimethylpyrrol-5-yl)methylidenyl]-2-indolinon
- CAS:194413-58-6
- MF:C15H14N2O
- Structure:
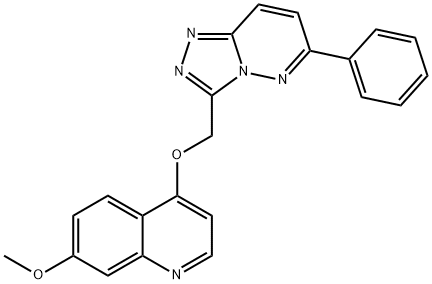
- Chemical Name:AMG-208
- CAS:1002304-34-8
- MF:C22H17N5O2
- Structure:
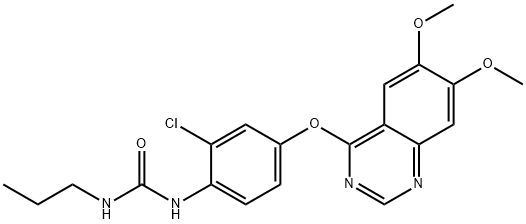
- Chemical Name:KRN 633
- CAS:286370-15-8
- MF:C20H21ClN4O4
- Structure:
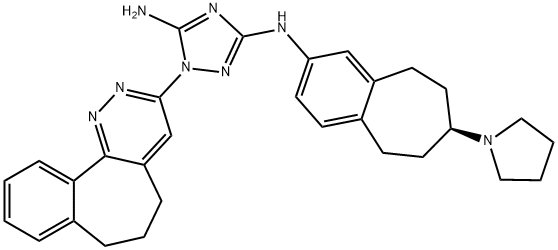
- Chemical Name:R428
- CAS:1037624-75-1
- MF:C30H34N8
- Structure:
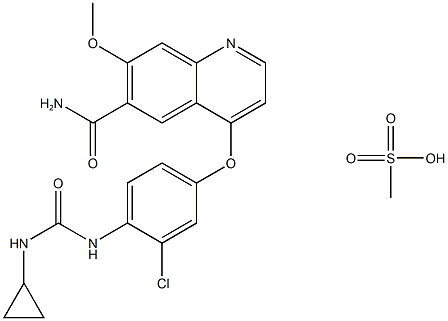
- Chemical Name:lenvatinib Mesylate
- CAS:857890-39-2
- MF:C22H23ClN4O7S
- Structure:
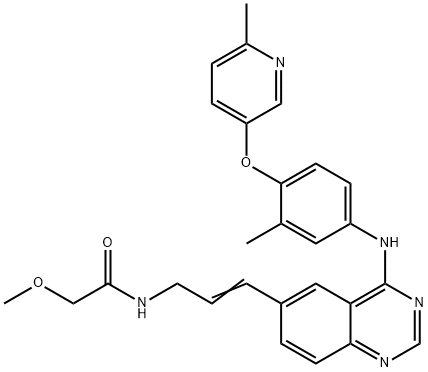
- Chemical Name:CP-724714
- CAS:537705-08-1
- MF:C27H27N5O3
- Structure:
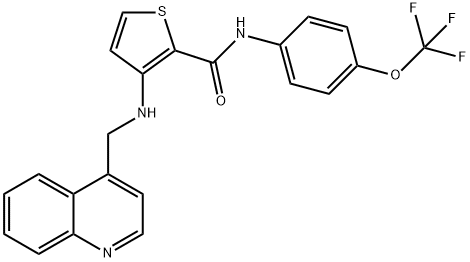
- Chemical Name:OSI-930
- CAS:728033-96-3
- MF:C22H16F3N3O2S
- Structure:
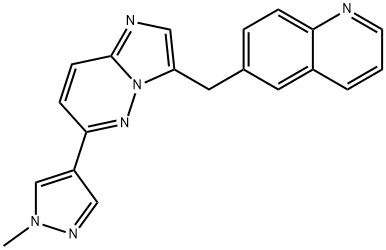
- Chemical Name:NVP-BVU 972
- CAS:1185763-69-2
- MF:C20H16N6
- Structure:
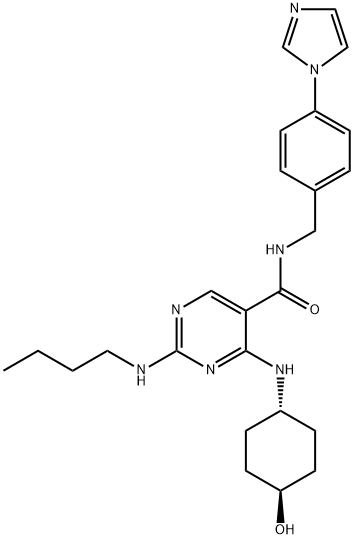
- Chemical Name:UNC2881
- CAS:1493764-08-1
- MF:C25H33N7O2
- Structure:
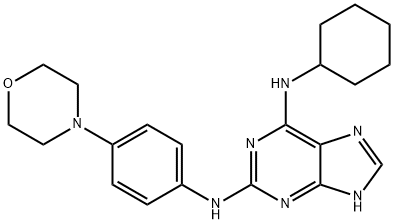
- Chemical Name:REVERSINE
- CAS:656820-32-5
- MF:C21H27N7O
- Structure:
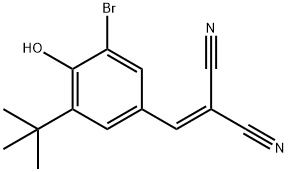
- Chemical Name:TYRPHOSTIN AG 1024
- CAS:65678-07-1
- MF:C14H13BrN2O
- Structure:
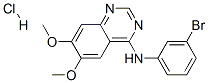
- Chemical Name:PD153035 HCl
- CAS:183322-45-4
- MF:C16H14BrN3O2.HCl
- Structure:
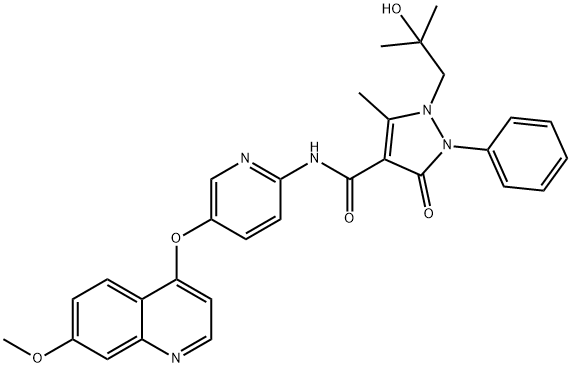
- Chemical Name:AMG 458
- CAS:913376-83-7
- MF:C30H29N5O5
- Structure:
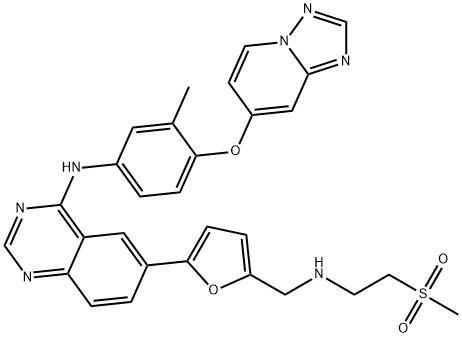
- Chemical Name:ARRY-380
- CAS:937265-83-3
- MF:C29H27N7O4S
- Structure:
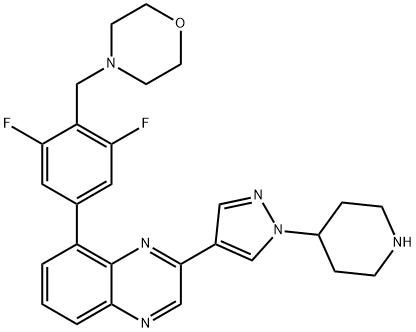
- Chemical Name:NVP-BSK805
- CAS:1092499-93-8
- MF:C27H28F2N6O
- Structure:
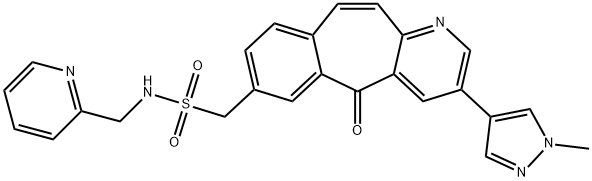
- Chemical Name:3-(1-Methyl-1H-pyrazol-4-yl)-5-oxo-N-(2-pyridinylmethyl)-5H-benzo[4,5]cyclohepta[1,2-b]pyridine-7-methanesulfonamide
- CAS:1001917-37-8
- MF:C25H21N5O3S
- Structure:
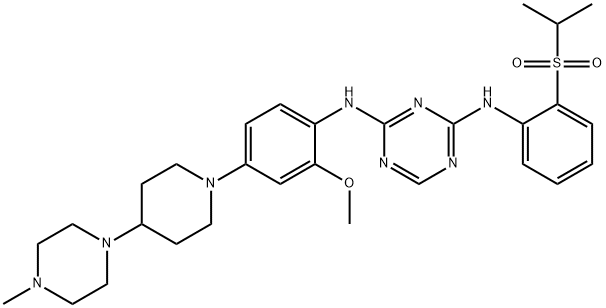
- Chemical Name:N2-[2-Methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl]phenyl]-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-1,3,5-triazine-2,4-diamine
- CAS:1097917-15-1
- MF:C29H40N8O3S
- Structure:
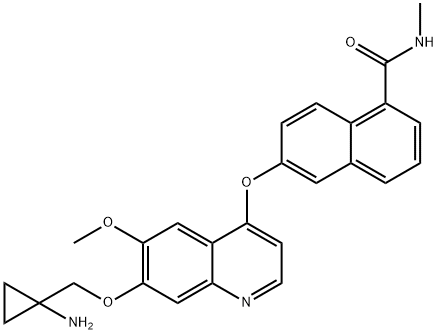
- Chemical Name:E-3810
- CAS:1058137-23-7
- MF:C26H25N3O4
- Structure:
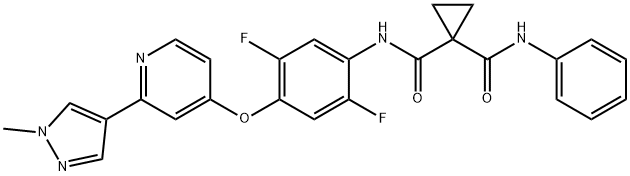
- Chemical Name:DCC-2618
- CAS:1225278-16-9
- MF:C26H21F2N5O3
- Structure:
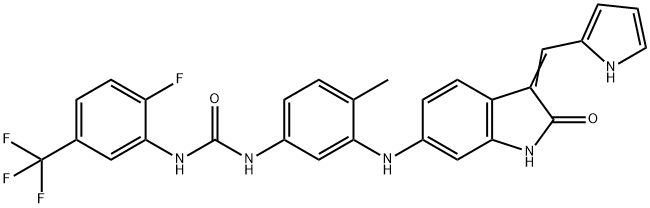
- Chemical Name:GNF 5837
- CAS:1033769-28-6
- MF:C28H21F4N5O2
- Structure:
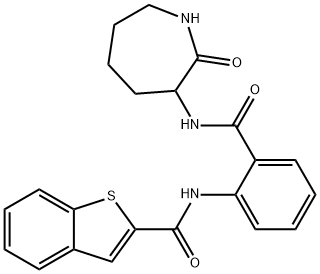
- Chemical Name:ANA12
- CAS:219766-25-3
- MF:C22H21N3O3S
- Structure:
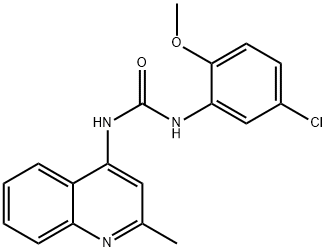
- Chemical Name:IGF-1R Inhibitor II, N-(5-Chloro-2-methoxyphenyl)-Nμ-(2-methylquinolin-4-yl)urea
- CAS:196868-63-0
- MF:C18H16ClN3O2
- Structure:
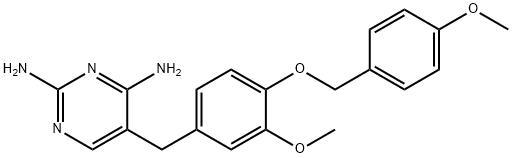
- Chemical Name:5-[3-Methoxy-4-(4-methoxy-benzyloxy)-benzyl]-pyrimidine-2,4-diamine
- CAS:870483-87-7
- MF:C20H22N4O3
- Structure:
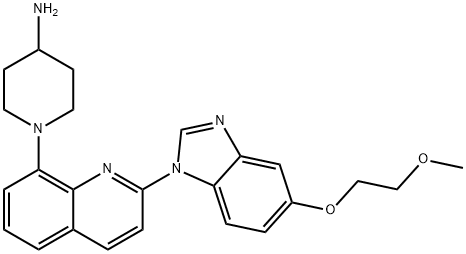
- Chemical Name:1-{2-[5-(2-Methoxy-ethoxy)-benzoimidazol-1-yl]-quinolin-8-yl}-piperidin-4-ylamine
- CAS:343787-29-1
- MF:C24H27N5O2
- Structure:
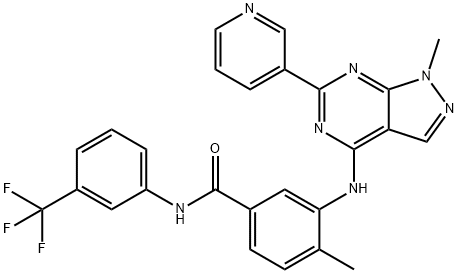
- Chemical Name:NVP-BHG712
- CAS:940310-85-0
- MF:C26H20F3N7O
- Structure:
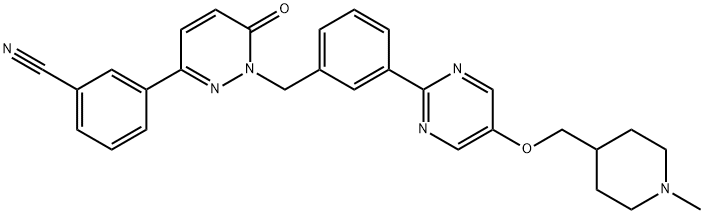
- Chemical Name:Tepotinib
- CAS:1100598-32-0
- MF:C29H28N6O2
- Structure:
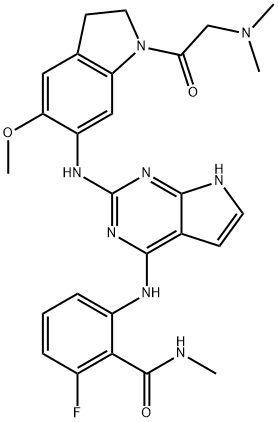
- Chemical Name:GSK1838705A
- CAS:1116235-97-2
- MF:C27H29FN8O3
- Structure:
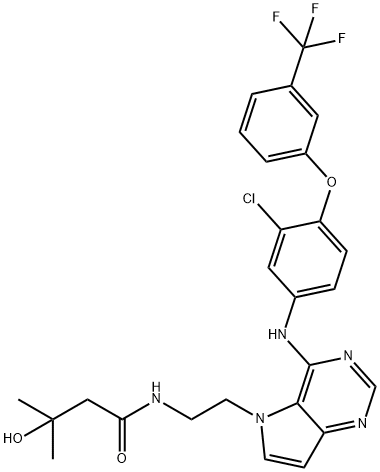
- Chemical Name:TAK-285
- CAS:871026-44-7
- MF:C26H25ClF3N5O3
- Structure:
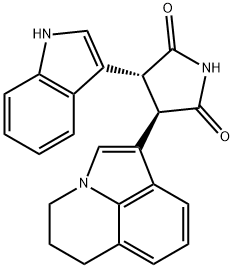
- Chemical Name:3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
- CAS:905854-02-6
- MF:C23H19N3O2
- Structure:
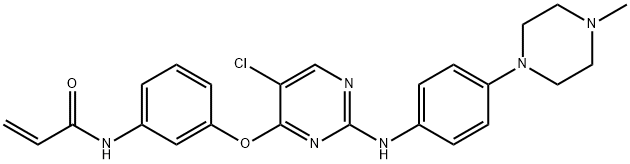
- Chemical Name:WZ3146
- CAS:1214265-56-1
- MF:C24H25ClN6O2
- Structure:
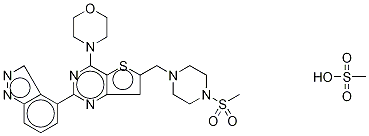
- Chemical Name:GDC-0941 Bimesylate
- CAS:957054-33-0
- MF:C24H30N7O6S3
- Structure:
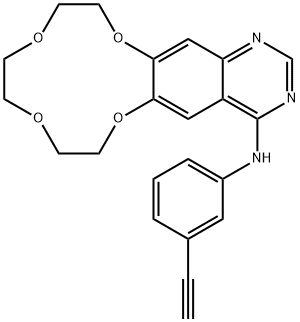
- Chemical Name:Icotinib
- CAS:610798-31-7
- MF:C22H21N3O4
- Structure:
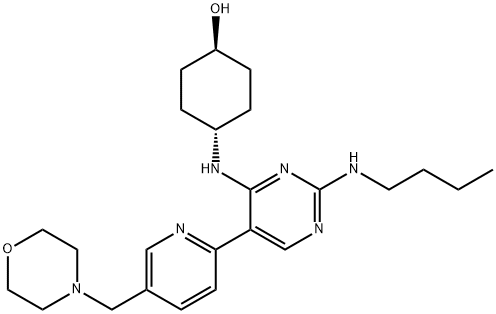
- Chemical Name:UNC2250
- CAS:1493694-70-4
- MF:C24H36N6O2
- Structure:

- Chemical Name:PD168393
- CAS:194423-15-9
- MF:C17H13BrN4O
- Structure:
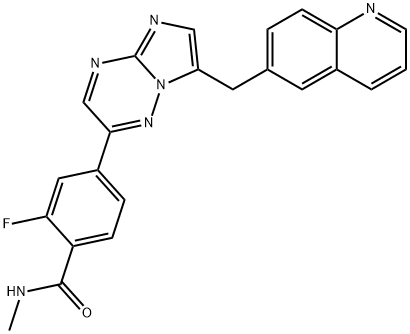
- Chemical Name:Capmatinib
- CAS:1029712-80-8
- MF:C23H17FN6O
- Structure:
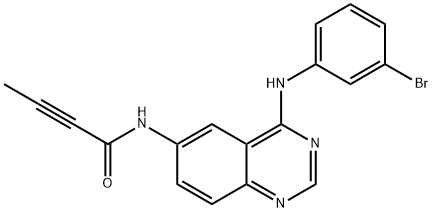
- Chemical Name:2-ButynaMide, N-[4-[(3-broMophenyl)aMino]-6-quinazolinyl]-
- CAS:194423-06-8
- MF:C18H13BrN4O
- Structure:
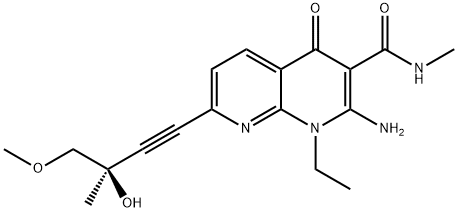
- Chemical Name:SAR 131675
- CAS:1433953-83-3
- MF:C18H22N4O4
- Structure:
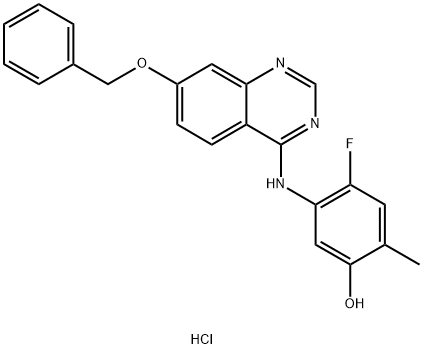
- Chemical Name:ZM 323881 hydrochloride
- CAS:193000-39-4
- MF:C22H19ClFN3O2
- Structure:
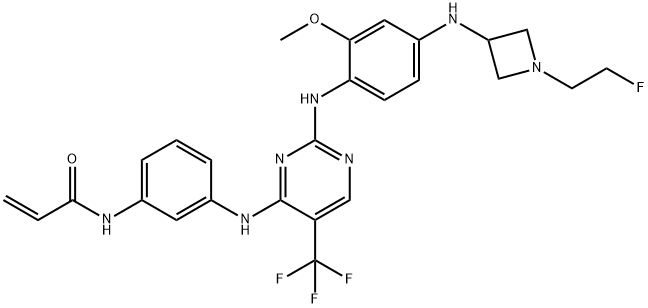
- Chemical Name:CNX-2006
- CAS:1375465-09-0
- MF:C26H27F4N7O2
- Structure:
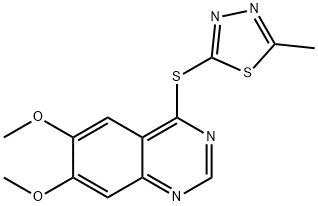
- Chemical Name:SKLB1002
- CAS:1225451-84-2
- MF:C13H12N4O2S2