Electrolyte Balance Pharmacology
Body fluid is generally bounded by the membrane, and divided into the intracellular fluid and extracellular fluid. And then the extracellular fluid is separated into the plasma and extracellular fluid with the capillaries as boundary. For the normal adult, the body fluid accounts about 60% of the body weight, in which the intracellular fluid accounts 40%, plasma 5%, and intercellular fluid 15%.
The solute in body fluid can be divided into two types : electrolyte and non-electrolyte. The non-electrolytic includes glucose and urea, while the electrolytes mainly contains cations like Na+, K+, Ca2+, Mg2+ and anions such as Cl-, HCO3-, HPO42-. There is a significant electrolyte distributed differences in and out of the cells. The extracellular fluid represented by the plasma mainly contains cations Na+, and anions Cl-, HCO3-.In the intracellular fluid ,the cations are mainly the K + and followed by the Mg2+, and the anions are mostly HPO42- and protein. The Na+-K+-ATP enzyme on the cell membrane can continue to pump the Na+ out of the cell, and the K+ into the cell, which is powered by the ATP.
The Na+ balance in and out of the cells needs 1 hour, and in the body takes 24 hours; while the K + requires 15 hours to reach the equilibrium between the inside and outside of the cells, and the equilibration time of the sick should be extended: the cardiopath needs 45 hours to reach the equilibrium. In addition, the cell entry of the K+ is accompanied by the addition of the glycogen and protein synthesis, otherwise the K+ escape from the cells. Therefore, the one-off clinical measurement of the K+ can not accurately reflect its current levels in the body. The K+ inside the cells recover slowly, which normally takes 4-6 days to reach the equilibrium, and for the serious patients ,it needs 10 to 20 days or even longer time to make the K+ inside the cells corrected. So the supplement of the K+ should follow the principle of slowness, low-concentration and fewness.
The main factors that determine the distribution of water between the body fluid is the body fluid osmolality. Osmolality is the attraction of the electrolyte and non-electrolyte solute particles to water. Normal osmotic pressure between plasma and cellular approximately equal to 280 ~ 320mmol / L. The solution that has equal osmolality with the plasma is called as the isotonic solution, and below or above this range is called as the hypotonic or hypertonic solution. Water can move from the hypotonic solution to the hypertonic solution through the cell membrane. Water and electrolyte disorder primarily affects the osmotic pressure and volume of the extracellular fluid. So the osmolality and volume changes of the body fluid are clinically calculated by the concentration change of the serum sodium, such as the sodium loses in proportion to the water for the isotonic water loss (dehydration); the loss of sodium is more than water for the hypotonic aqueous loss; the loss of water is more than sodium for the hypertonic dehydration.
- Structure:
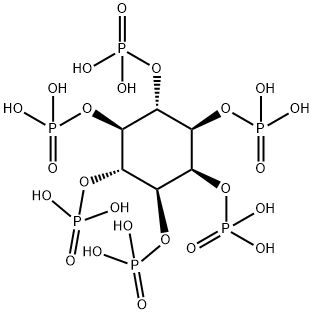
- Chemical Name:Phytic acid
- CAS:83-86-3
- MF:C6H18O24P6
- Structure:
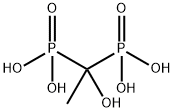
- Chemical Name:Etidronic Acid
- CAS:2809-21-4
- MF:C2H8O7P2
- Structure:
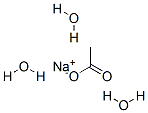
- Chemical Name:Sodium acetate trihydrate
- CAS:6131-90-4
- MF:C2H9NaO5
- Structure:

- Chemical Name:Calcium chloride
- CAS:10043-52-4
- MF:CaCl2
- Structure:
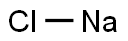
- Chemical Name:Sodium chloride
- CAS:7647-14-5
- MF:ClNa
- Structure:
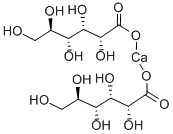
- Chemical Name:Calcium gluconate
- CAS:299-28-5
- MF:C12H22CaO14
- Structure:
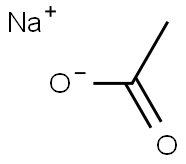
- Chemical Name:Sodium acetate
- CAS:127-09-3
- MF:C2H3NaO2
- Structure:
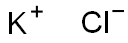
- Chemical Name:Potassium chloride
- CAS:7447-40-7
- MF:KCl
- Structure:
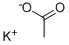
- Chemical Name:Potassium Acetate
- CAS:127-08-2
- MF:C2H3KO2
- Structure:
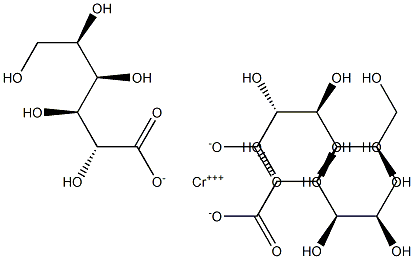
- Chemical Name:chromium gluconate
- CAS:
- MF:C18H33CrO21
- Chemical Name:Salmon calcitonin
- CAS:
- MF:
- Structure:
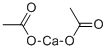
- Chemical Name:Calcium acetate
- CAS:62-54-4
- MF:C4H6CaO4
- Structure:
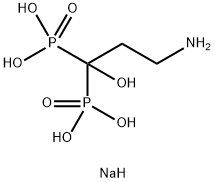
- Chemical Name:Pamidronate disodium salt
- CAS:57248-88-1
- MF:C3H12NNaO7P2
- Structure:
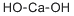
- Chemical Name:Calcium hydroxide
- CAS:1305-62-0
- MF:CaH2O2
- Structure:
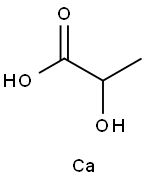
- Chemical Name:Calcium lactate
- CAS:814-80-2
- MF:C3H8CaO3
- Structure:
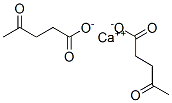
- Chemical Name:CALCIUM LEVULINATE
- CAS:591-64-0
- MF:C10H14CaO6
- Structure:
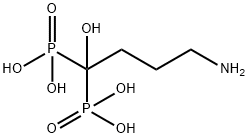
- Chemical Name:Alendronic acid
- CAS:66376-36-1
- MF:C4H13NO7P2
- Structure:
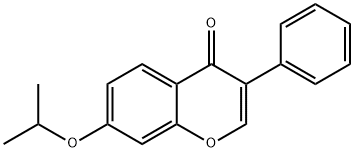
- Chemical Name:Ipriflavone
- CAS:35212-22-7
- MF:C18H16O3
- Structure:
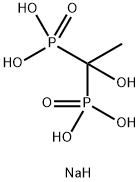
- Chemical Name:Etidronate disodium
- CAS:7414-83-7
- MF:C2H9NaO7P2
- Structure:
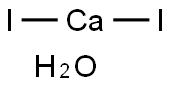
- Chemical Name:CALCIUM IODIDE
- CAS:10031-31-9
- MF:CaH2I2O
- Structure:

- Chemical Name:IBANDRONATE
- CAS:
- MF:C9H23NO7P2
- Structure:
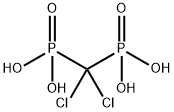
- Chemical Name:CLODRONIC ACID
- CAS:10596-23-3
- MF:CH4Cl2O6P2
- Structure:
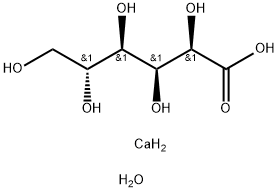
- Chemical Name:CALCIUM GLUCONATE MONOHYDRATE
- CAS:66905-23-5
- MF:C6H16CaO8
- Structure:
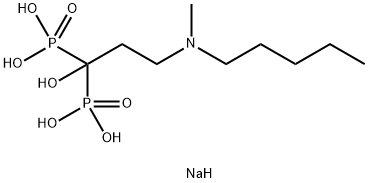
- Chemical Name:Ibandronate sodium
- CAS:138844-81-2
- MF:C9H24NNaO7P2
- Structure:
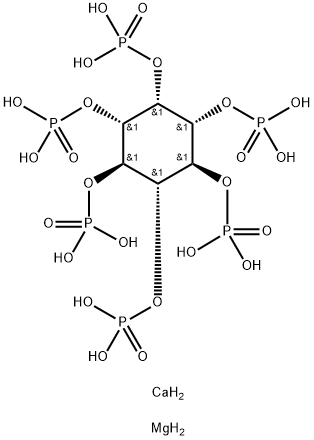
- Chemical Name:Calcium phytate
- CAS:3615-82-5
- MF:C6H22CaMgO24P6
- Structure:
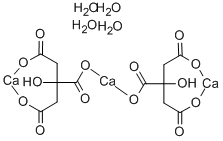
- Chemical Name:Calcium citrate tetrahydrate
- CAS:5785-44-4
- MF:C12H18Ca3O18
- Structure:
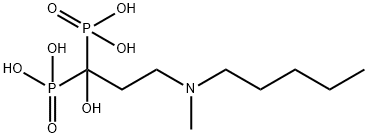
- Chemical Name:Ibandronic acid
- CAS:114084-78-5
- MF:C9H23NO7P2
- Structure:
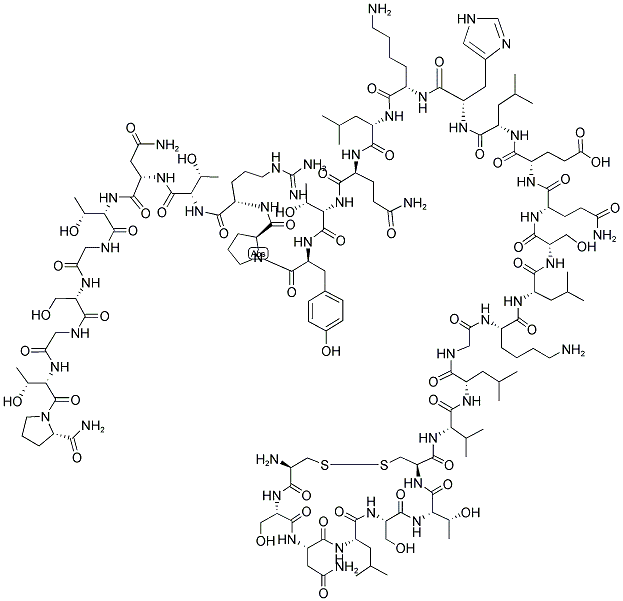
- Chemical Name:Calcitonin
- CAS:9007-12-9
- MF:C145H240N44O48S2
- Structure:
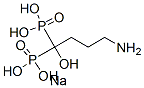
- Chemical Name:Alendronate sodium
- CAS:129318-43-0
- MF:C4H13NNaO7P2
- Structure:
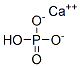
- Chemical Name:Calcium phosphate dibasic
- CAS:7757-93-9
- MF:CaHO4P
- Structure:
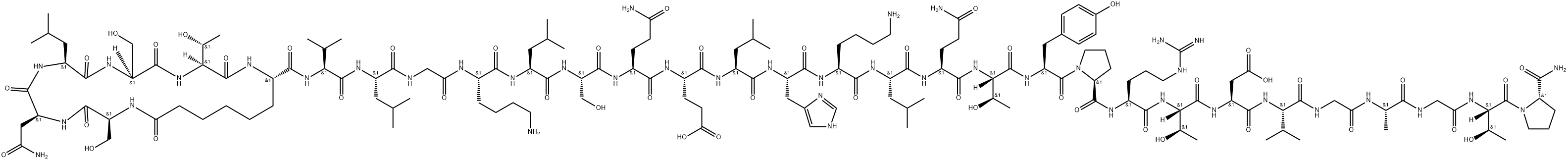
- Chemical Name:Elcatonin
- CAS:60731-46-6
- MF:C148H244N42O47
- Structure:
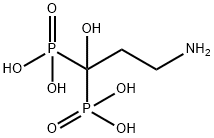
- Chemical Name:Pamidronic acid
- CAS:40391-99-9
- MF:C3H11NO7P2
- Structure:
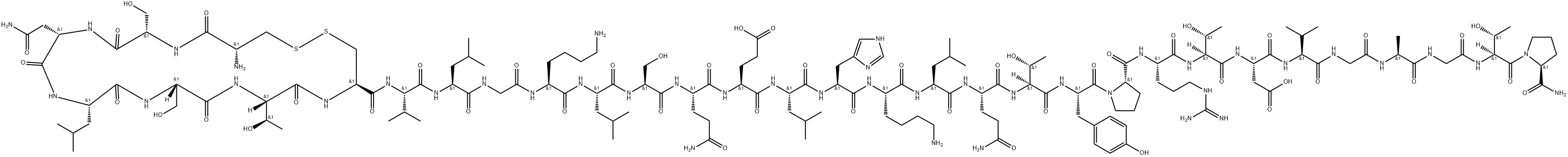
- Chemical Name:Calcitonin eel
- CAS:57014-02-5
- MF:C146H241N43O47S2
- Structure:
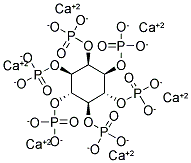
- Chemical Name:PHYTIN
- CAS:7776-28-5
- MF:C6H6Ca6O24P6
- Chemical Name:CLODRONATE
- CAS:
- MF:
- Structure:
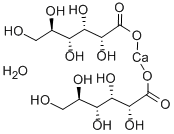
- Chemical Name:CALCIUM GLUCONATE MONOHYDRATE
- CAS:18016-24-5
- MF:C12H24CaO15
- Chemical Name:Active Calcium
- CAS:
- MF:
- Chemical Name:PolystyreneSulronate
- CAS:
- MF:
- Chemical Name:Oyster calcium
- CAS:
- MF:
- Structure:
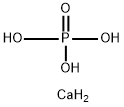
- Chemical Name:Phosphoric acid, calcium salt (1:)
- CAS:10103-46-5
- MF:CaH5O4P