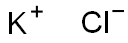Physical properties
Muriate of potash is a metal halide salt, it is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste.

Uses
Muriate of potash is used as a fertilizer, in medicine, in scientific applications, domestic water softeners (as a substitute for sodium chloride salt), and in food processing, where it may be known as E number additive E508.
Preparation
Muriate of potash is inexpensively available and is rarely prepared intentionally in the laboratory. It can be generated by treating potassium hydroxide (or other potassium bases) with hydrochloric acid:
KOH+HCl⟶KCl+H2O
Health Hazard
Muriate of potash is an essential constituent of the body for intracellular osmotic pressure and buffering, cell permeability, acid-base balance, muscle contraction and nerve function.
SYMPTOMS: Large doses of Muriate of potash usually induce vomiting, so acute intoxication by mouth is rare. If no pre-existing kidney damage, it is rapidly excreted. Poisoning disturbs the rhythm of heart. Large doses by mouth can cause gastrointestinal irritation, purging, weakness, and circulatory disturbances.
Chemical Reactivity
Muriate of potash is not in general strongly reactive. Violent reaction with BrF3 and with a mixture of sulfuric acid potassium permanganate mixture . Reacts with concentrated sulfuric acid to generate fumes of hydrogen chloride.
Toxicity
The typical amounts of muriate of potash found in the diet appear to be generally safe.In larger quantities, however, muriate of potash is toxic. The LD50 of orally ingested kcl is approximately 2.5 g/kg, or 190 grams (6.7 oz) for a body mass of 75 kilograms (165 lb).