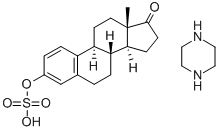
Estra-1,3,5(10)-trien-17-on-3-sulfat, Verbindung mit Piperazin (1:1)
Bezeichnung:Estra-1,3,5(10)-trien-17-on-3-sulfat, Verbindung mit Piperazin (1:1)
CAS-Nr7280-37-7
Englisch Name:Estropipate
CBNumberCB8331281
SummenformelC22H32N2O5S
Molgewicht436.56
MOL-Datei7280-37-7.mol
Synonyma
Estra-1,3,5(10)-trien-17-on-3-sulfat, Verbindung mit Piperazin (1:1)
Estra-1,3,5(10)-trien-17-on-3-sulfat, Verbindung mit Piperazin (1:1) physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 245 °C |
| alpha | D25 +87.8° (c = 1 in 0.4% NaOH) |
| storage temp. | 2-8°C |
| Löslichkeit | DMSO: ≥24mg/mL |
| pka | pKa 3.6/9.7(acetonitrile,80% v/v) (Uncertain) |
| Aggregatzustand | powder |
| Farbe | white to tan |
| Optische Aktivität | [α]/D 100 to 120°, c = 1 in DMSO |
| CAS Datenbank | 7280-37-7(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 45-22 |
| S-Sätze: | 53-45 |
| WGK Germany | 3 |
| RTECS-Nr. | KG7785000 |
| HS Code | 2937230000 |


