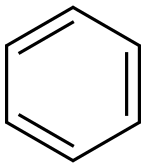
Benzol
Bezeichnung:Benzol
CAS-Nr71-43-2
Englisch Name:Benzene
CBNumberCB6854153
SummenformelC6H6
Molgewicht78.11
MOL-Datei71-43-2.mol
Synonyma
Benzol
Cyclohexatrien
Benzen
Benzol physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 5.5 °C (lit.) |
| Siedepunkt | 80 °C (lit.) |
| Dichte | 0.874 g/mL at 25 °C (lit.) |
| Dampfdichte | 2.77 (vs air) |
| Dampfdruck | 166 mm Hg ( 37.7 °C) |
| Brechungsindex | n |
| Flammpunkt | 12 °F |
| storage temp. | room temp |
| Löslichkeit | Miscible with alcohol, chloroform, dichloromethane, diethyl ether, acetone and acetic acid. |
| pka | 43(at 25℃) |
| Aggregatzustand | Liquid |
| Farbe | APHA: ≤10 |
| Relative polarity | 0.111 |
| Geruch (Odor) | Paint-thinner-like odor detectable at 12 ppm |
| Odor Threshold | 2.7ppm |
| Explosionsgrenze | 1.4-8.0%(V) |
| Wasserlöslichkeit | 0.18 g/100 mL |
| maximale Wellenlänge (λmax) | λ: 280 nm Amax: 1.0 λ: 290 nm Amax: 0.15 λ: 300 nm Amax: 0.06 λ: 330 nm Amax: 0.02 λ: 350-400 nm Amax: 0.01 |
| Merck | 14,1066 |
| BRN | 969212 |
| Henry's Law Constant | 10.4 at 45.00 °C, 11.4 at 50.00 °C, 13.3 at 55.00 °C, 14.5 at 60.00 °C, 16.8 at 65.00 °C, 19.2 at 70.00 °C (static headspace-GC, Park et al., 2004) |
| Expositionsgrenzwerte | TLV-TWA 10 ppm (~32 mg/m3) (ACGIH and OSHA); ceiling 25 ppm (~80 mg/m3) (OSHA and MSHA); peak 50 ppm (~160 mg/m3)/10 min/8 h (OSHA); carcinogenicity: Suspected Human Carcinogen (ACGIH), Human Sufficient Evidence (IARC). |
| Dielectric constant | 2.3(20℃) |
| Stabilität | Stable. Substances to be avoided include strong oxidizing agents, sulfuric acid, nitric acid, halogens. Highly flammable. |
| InChIKey | UHOVQNZJYSORNB-UHFFFAOYSA-N |
| LogP | 2.130 |
| CAS Datenbank | 71-43-2(CAS DataBase Reference) |
| IARC | 1 (Vol. 29, Sup 7. 100F, 120) 2018 |
| NIST chemische Informationen | Benzene(71-43-2) |
| EPA chemische Informationen | Benzene (71-43-2) |
| Kennzeichnung gefährlicher | F,T |
| R-Sätze: | 45-46-11-36/38-48/23/24/25-65-39/23/24/25-23/24/25 |
| S-Sätze: | 53-45-36/37-16-7 |
| RIDADR | UN 1114 3/PG 2 |
| OEB | E |
| OEL | TWA: 0.1 ppm, STEL: 1 ppm |
| WGK Germany | 3 |
| RTECS-Nr. | CY1400000 |
| F | 3-10 |
| Selbstentzündungstemperatur | 560 °C |
| TSCA | Yes |
| HS Code | 2902 20 00 |
| HazardClass | 3 |
| PackingGroup | II |
| Giftige Stoffe Daten | 71-43-2(Hazardous Substances Data) |
| Toxizität | LD50 orally in young adult rats: 3.8 ml/kg (Kimura) |
| IDLA | 500 ppm |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)



-
Alarmwort
Achtung
-
Gefahrenhinweise
H225:Flüssigkeit und Dampf leicht entzündbar.
H304:Kann bei Verschlucken und Eindringen in die Atemwege tödlich sein.
H315:Verursacht Hautreizungen.
H319:Verursacht schwere Augenreizung.
H340:Kann genetische Defekte verursachen.
H350:Kann Krebs verursachen.
H372:Schädigt bei Hautkontakt und Verschlucken die Organe bei längerer oder wiederholter Exposition.
H412:Schädlich für Wasserorganismen, mit langfristiger Wirkung.
-
Sicherheit
P210:Von Hitze, heißen Oberflächen, Funken, offenen Flammen und anderen Zündquellenarten fernhalten. Nicht rauchen.
P273:Freisetzung in die Umwelt vermeiden.
P301+P310:BEI VERSCHLUCKEN: Sofort GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P303+P361+P353:BEI BERÜHRUNG MIT DER HAUT (oder dem Haar): Alle kontaminierten Kleidungsstücke sofort ausziehen. Haut mit Wasser abwaschen oder duschen.
P305+P351+P338:BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen.
P331:KEIN Erbrechen herbeiführen.
Benzene Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
FARBLOSE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH. -
PHYSIKALISCHE GEFAHREN
Die Dämpfe sind schwerer als Luft und können sich am Boden ausbreiten. Fernzündung möglich. Fließen, Schütten o.ä. kann zu elektrostatischer Aufladung führen. -
CHEMISCHE GEFAHREN
Reagiert heftig mit Oxidationsmitteln, Salpetersäure, Schwefelsäure und Halogenen. Feuer- und Explosionsgefahr! Greift Kunststoff und Gummi an. -
ARBEITSPLATZGRENZWERTE
TLV: 0.5 ppm (als TWA); 2.5 ppm (als STEL); Hautresorption; Krebskategorie A1(bestätigte krebserzeugende Wirkung beim Menschen); BEI vorhanden (ACGIH 2005).
MAK: Hautresorption; Krebserzeugend Kategorie 1; Keimzellmutagen Kategorie 3A; (DFG 2005).
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation, über die Haut und durch Verschlucken. -
INHALATIONSGEFAHREN
Beim Verdampfen dieser Substanz bei 20 °C tritt sehr schnell eine gesundheitsschädliche Kontamination der Luft ein. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz reizt die Augen, die Haut und die Atmungsorgane. Verschlucken kann zur Aufnahme in der Lunge führen; Gefahr der Aspirationspneumonie. Möglich sind Auswirkungen auf das zentrale Nervensystem mit nachfolgender Bewusstseinstrübung. Exposition weit oberhalb der Arbeitsplatzgrenzwerte kann zu Bewusstlosigkeit und zum Tod führen. -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Die Flüssigkeit entfettet die Haut. Möglich sind Auswirkungen auf Knochenmarkund Immunsystem. Führt zu Abnahme der Blutzellen. Krebserzeugend für den Menschen. -
LECKAGE
Zündquellen entfernen. Ausgelaufene Flüssigkeit möglichst in abdichtbaren Behältern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. NICHT in die Kanalisation spülen! NICHT in die Umwelt gelangen lassen. Persönliche Schutzausrüstung: Vollschutzanzug mit umgebungsluftunabhängigem Atemschutzgerät. -
R-Sätze Betriebsanweisung:
R45:Kann Krebs erzeugen.
R46:Kann vererbbare Schäden verursachen.
R11:Leichtentzündlich.
R36/38:Reizt die Augen und die Haut.
R48/23/24/25:Giftig: Gefahr ernster Gesundheitsschäden bei längerer Exposition durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R65:Gesundheitsschädlich: kann beim Verschlucken Lungenschäden verursachen.
R39/23/24/25:Giftig: ernste Gefahr irreversiblen Schadens durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R23/24/25:Giftig beim Einatmen, Verschlucken und Berührung mit der Haut. -
S-Sätze Betriebsanweisung:
S53:Exposition vermeiden - vor Gebrauch besondere Anweisungen einholen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen. -
Aussehen Eigenschaften
C6H6. Wasserunlösliche, farblose, charakteristisch riechende Flüssigkeit. -
Gefahren für Mensch und Umwelt
Leichtentzündlich. Gefährliche Reaktionen mit starken Oxidationsmitteln oder mit Leichtmetallen in Gegenwart von Chlorkohlenwasserstoffen.
Giftig beim Einatmen, Verschlucken und bei Berührung mit der Haut. Gefahr ernster Gesundheitsschäden bei längerer Exposition.
Benzol zeigt bei akuter oder chronischer Aufnahme unterschiedliche Wirkungen: Höhere Dampfkonzentationen führen zu einer starken Depression des zentralen Nervensystems, zunächst treten jedoch Euphorie und Erregung auf. Danach folgen je nach Konzentration und Einwirkungszeit Kopfschmerzen, Schwindel, unregelmäßiges Sprechen und auch Bewußtlosigkeit. Höhere Konzentrationen sind nach kurzer Zeit tödlich. Bei rechtzeitiger Frischluftzufuhr bleiben keine Schäden zurück. Flüssiges Benzol reizt die Haut und Schleimhäute und wirkt entfettend auf die Haut. Benzol wird leicht durch die Haut aufgenommen! Bei Verschlucken folgen Magenschmerzen und bei verzögerter Entfernung auch Störungen des zentralen Nervensystems.
Die chronische Einwirkung führt nach langer, symptomfreierLatenzzeit zu Schädigungen der Blutbildungsorgane und Bildung von Krebsgewebe.
Stark wassergefährdender Stoff (WGK 3). -
Schutzmaßnahmen und Verhaltensregeln
Im Abzug arbeiten! Exposition vermeiden. Von Zündquellen fernhalten. Wenn möglich, Ersatzstoffe verwenden.
Schutzhandschuhe aus Latex und Neopren sind nicht gegen Benzol beständig (nur als sehr kurzzeitiger Spritzschutz verwendbar). -
Verhalten im Gefahrfall
Kleine Spritzer im Abzug verdampfen lassen, größere Mengen mit Absorptionsmaterial (Rench-Rapid) aufnehmen und entsorgen. Dämpfe nicht einatmen. Atemschutz: Kombinationsfilter ABEK.
Brände mit CO2-Löscher bekämpfen! -
Erste Hilfe
Nach Hautkontakt: Mit viel Wasser und Seife abwaschen.
Nach Augenkontakt: Mit Wasser mindestens 15 Minuten bei geöffnetem Lidspalt ausspülen. Augenarzt!
Nach Einatmen: Frischluft. Arzt!
Nach Verschlucken: Paraffinöl verabreichen. Erbrechen vermeiden. Arzt!
Nach Kleidungskontakt: Kontaminierte Kleidung sofort ausziehen.
Ersthelfer: siehe gesonderten Anschlag -
Sachgerechte Entsorgung
Als Sondermüll (halogenfreie Lösungsmittel) entsorgen. -
Beschreibung
Benzene is a colorless, volatile, highly flammable liquid that is used extensively in the chemical industry and received wide interest in the early days of organic chemistry.

Because of its structure, benzene is a very stable organic compound. It does not readily undergo addition reactions. Addition reactions involving benzene require high temperature, pressure, and special catalysts. The most common reactions involving benzene involve substitution reactions. Numerous atoms and groups of atoms may replace a hydrogen atom or several hydrogen atoms in benzene. Th ree important types of substitution reactions involving benzene are alkylation, halogenation, and nitration. In alkylation, an alkyl group or groups substitute for hydrogen(s). -
Chemische Eigenschaften
Benzene is a clear, volatile, colorless, highly flammable liquid with a pleasant, characteristic odor. It is an aromatic hydrocarbon that boils at 80.1 DC. Benzene is used as a solvent in many areas of industries, such as rubber and shoe manufacturing, and in the production of other important substances, such as styrene, phenol, and cyclohexane. It is essential in the manufacture of detergents, pesticides, solvents, and paint removers. It is present in fuels such as gasoline up to the level of 5%. -
Physikalische Eigenschaften
Clear, colorless to light yellow watery liquid with an aromatic, musty, phenolics or gasoline-like odor. At 40 °C, an odor threshold concentration of 190 μg/L in air was determined by Young et al. (1996). An odor threshold of 4.68 ppmv was determined by Leonardos et al. (1969). A detection odor threshold concentration of 108 mg/m3 (34 ppmv) was reported by Punter (1983). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 0.072 and 0.5 mg/L, respectively (Alexander et al., 1982). -
Occurrence
Detectable levels of benzene have been found in a number of soft drinks that contain either a sodium or potassium benzoate preservative and ascorbic acid, and 'diet' type products containing no added sugar are reported to be particularly likely to contain benzene at detectable levels. Surveys carried out in the USA, the UK and Canada have all confirmed that a small proportion of these products may contain low levels of benzene. For example, in a survey of 86 samples analysed by the FDA between April 2006 and March 2007, only five products were found to contain benzene at concentrations above 5 ug kg-1. The levels found were in a range from approximately 10–90 ug kg-1. A survey of 150 UK-produced soft drinks by the Food Standards Agency (FSA) published in 2006 showed that four products contained benzene at levels above 10 ug kg-1, and the highest level recorded was 28 ug kg-1. However, it has been reported that higher levels may develop in these products during prolonged storage, especially if they are exposed to daylight.
Benzene may also be formed in some mango and cranberry drinks in the absence of added preservatives, because these fruits contain natural benzoates. -
History
Benzene was discovered in 1825 by Michael Faraday (1791–1867), who identified it in a liquid residue from heated whale oil. Faraday called the compound bicarburet of hydrogen, and its name was later changed to benzin by Eilhardt Mitscherlich (1794–1863), who isolated the compound from benzoin (C14H12O2). -
Verwenden
Benzene is also converted to cyclohexane, which is used to produce nylon and synthetic fibers. -
Verwenden
Benzene occurs in coal and coal-tar distillationproducts and in petroleum products suchas gasoline. It is also found in the gases andleachates of landfills for industrial wastes,construction debris, and landscaping refuse(Oak Ridge National Laboratory 1989). Traceamounts of benzene, toluene, xylenes, andother volatile organics have been found inthe soils and groundwaters near many sanitarylandfills (U.S. EPA 1989a,b). Kramer(1989) has assessed the level of exposuresto benzene during removal, cleaning, pumping,and testing of underground gasoline storagetanks. The average human exposureswere 0.43–3.84 ppm (in 1.5–6 hours) and thehighest short-term (15–minute) exposure was9.14 ppm. Benzene also occurs in the tobaccosmoke (Hoffmann et al. 1989); thus the riskof its exposure may enhance from inhalingsuch smoke.
Benzene is used as a solvent for waxes,resins, and oils; as a paint remover; as a diluentfor lacquers; in the manufacture of dyes,pharmaceuticals, varnishes, and linoleum;and as a raw material to produce a numberof organic compounds. -
Verwenden
Benzene is also known as benzol, benzole, coal tar naphtha, and phenyl hydride, benzene is a clear, colorless, flammable liquid made by passing coke gas through oil, which is then distilled to produce benzene and toluol. The benzene is separated from the toluol by fractional distillation. Benzene is soluble in alcohol, ether, chloroform, and glacial acetic acid, but it is insoluble in water. Benzene was used as a solvent for many photographic operations in the 19th century. In the collodion process, benzene was used to dissolve rubber to both subcoat and supercoat negatives. It was also used as a solvent for Canada balsam in the Cutting method of sealing ambrotypes and cementing lens elements. Benzene was also used as a solvent for wax, gums, resins, and amber and in particular for retouching varnishes applied to silver bromide gelatin negatives. -
Verwenden
Manufacturing of ethylbenzene (for styrene monomer), dodecylbenzene (for detergents), cyclo- hexane (for nylon), phenol, nitrobenzene (for ani- line), maleic anhydride, chlorobenzene, diphenyl, benzene hexachloride, benzene-sulfonic acid, and as a solvent. -
Definition
ChEBI: Benzene is a six-carbon aromatic annulene in which each carbon atom donates one of its two 2p electrons into a delocalised pi system. A toxic, flammable liquid byproduct of coal distillation, it is used as an industrial solvent. Benzene is a carcinogen that also damages bone marrow and the central nervous system. -
Vorbereitung Methode
Today benzene, which is a natural component of petroleum, is obtained from petroleum by several processes. Toluene hydrodealkylation involves mixing toluene (C6H5CH3) and hydrogen in the presence of catalysts and temperatures of approximately 500°C and pressures of about 50 atmospheres to produce benzene and methane: C6H5CH3 + H2 → C6H6 + CH4. Hydrodealkylation strips the methyl group from toluene to produce benzene. Toluene disproportionation involves combining toluene so that the methyl groups bond to one aromatic ring, producing benzene and xylene. Benzene can also be obtained from petroleum reforming in which temperature, pressure, and catalysts are used to convert petroleum components to benzene, which can then be extracted using solvents and distillation processes. Another source of benzene is pyrolysis gasoline or pygas. -
Reaktionen
Benzene reacts (1) with chlorine, to form (a) substitution products (one-half of the chlorine forms hydrogen chloride) such as chlorobenzene, C6H5Cl; dichlorobenzene, C6H4Cl2(1,4) and (1,2); trichlorobenzene, C6H3Cl3(1,2,4); tetrachlorobenzene (1,2,3,5); and (b) addition products, such as benzene dichloride C6H6Cl2; benzene tetrachloride, C6H6Cl4; and benzene hexachloride, C6H6Cl6. The formation of substitution products of the benzene nucleus, whether in benzene or its homologues, is favored by the presence of a catalyzer, e.g., iodine, phosphorus, iron; (2) with concentrated HNO3, to form nitrobenzene, C6H5NO2; 1,3- dinitrobenzene, C6H4(NO2)2 (1,3), 1,3,5-trinitrobenzene, C6H3(NO2)3 (1,3,5); (3) with concentrated H2SO4, to form benzene sulfonic acid, C6H5SO3H, benzene disulfonic acid, C6H4(SO3H)2(1,3), benzene trisulfonic acid, C6H3(SO3H)3 (1,3–5); (4) with methyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form toluene, monomethyl benzene, C6H5CH3; dimethyl benzene C6H4(CH3)2; trimethyl benzene, C6H3(CH3)3; (5) with acetyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form acetophenone (methylphenyl ketone), C6H5COCH3. -
Allgemeine Beschreibung
Benzene appears as a clear colorless liquid with a petroleum-like odor. Flash point less than 0 °F. Less dense than water and slightly soluble in water. Hence floats on water. Vapors are heavier than air. -
Air & Water Reaktionen
Highly flammable. Slightly soluble in water. -
Reaktivität anzeigen
Benzene reacts vigorously with allyl chloride or other alkyl halides even at minus 70°C in the presence of ethyl aluminum dichloride or ethyl aluminum sesquichloride. Explosions have been reported [NFPA 491M 1991]. Ignites in contact with powdered chromic anhydride [Mellor 11:235 1946-47]. Incompatible with oxidizing agents such as nitric acid. Mixtures with bromine trifluoride, bromine pentafluoride, iodine pentafluoride, iodine heptafluoride and other interhalogens can ignite upon heating [Bretherick 5th ed. 1995]. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.). The reaction of Benzene and trichloroacetonitrile evolves toxic chloroform and HCl gasses. (Hagedorn, F., H.-P. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.). -
Hazard
The acute toxicity of benzene is low. Inhalation of benzene can cause dizziness, euphoria, giddiness, headache, nausea, drowsiness, and weakness. Benzene can cause moderate irritation to skin and severe irritation to eyes and mucous membranes. Benzene readily penetrates the skin to cause the same toxic effects as inhalation or ingestion. The chronic toxicity of benzene is significant. Exposure to benzene affects the blood and blood-forming organs such as the bone marrow, causing irreversible injury; blood disorders including anemia and leukemia may result. The symptoms of chronic benzene exposure may include fatigue, nervousness, irritability, blurred vision, and labored breathing. Benzene is regulated by OSHA as a carcinogen (Standard 1910.1028) and is listed in IARC Group 1 ("carcinogenic to humans"). This substance is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard. -
Health Hazard
Benzene is an acute as well as a chronictoxicant. The acute toxic effects from inhalation,ingestion, and skin contact are low tomoderate. The symptoms in humans are hallucination,distorted perception, euphoria, somnolence,nausea, vomiting, and headache. Thenarcotic effects in humans may occur frominhaling benzene in air at a concentrationof 200 ppm. High concentrations may cause convulsions. A 5- to 10-minute exposure to2% benzene in air may be fatal. Death mayresult from respiratory failure.
Benzene is an irritant to the eyes, nose,and respiratory tract. The chronic poisoningfrom benzene is much more severe than itsacute toxicity. The target organs to acuteand chronic poisoning are the blood, bonemarrow, central nervous system, respiratorysystem, eyes, and skin. Heavy occupationalexposures to benzene can cause bone marrowdepression and anemia, and in rare cases,leukemia. Leukemia may develop severalyears after the exposure ceases. Deaths fromleukemia, attributed to occupational exposureto benzene in the workplace, which may beon the order of 200 ppm concentration, havebeen documented (ACGIH 1986). Benzene islisted as a suspected human carcinogen. Inaddition to leukemia, malignant lymphoma,and myeloma, lung cancer in subjects exposedto benzene has been reported (Aksoy 1989).
Absorption of liquid benzene through theskin may be harmful. The main eliminationpathway for benzene absorbed throughinhalation or skin contact is metabolism.Hydroxyl radicals play an important role inthe process of metabolism. Khan and coworkers(1990) have reported the formationof formaldehyde and degradation of deoxyribose,suggesting the generation of hydroxylradicals during benzene toxicity to the bonemarrow S-9 fraction. The hydroxyl radicalsreact with benzene to form phenols and dihydroxyphenols,which are excreted rapidly inurine. About one-third of the retained benzeneis excreted as phenols in the urine. Theremaining two-thirds may be further degradedand attached onto the tissue or oxidized andexhaled as CO2.
Kalf and associates (1989) have investigatedthe action of prostaglandin H synthasein benzene toxicity and preventionof benzene-induced myelo- and genotoxicityby nonsteroidal anti-inflammatory drugs(NSAIDs). Indomethacin, a prostaglandin Hsynthase inhibitor prevented the dose- dependentbone marrow depression and increase in marrow prostaglandin E level in mice intravenouslydosed with benzene. Indomethacin,aspirin, or meclofenamate prevented thedecrease in cellularity and increase in micronucleatedpolychromatic erythrocytes in peripheralblood, caused by intravenous injectionof benzene (100–1000 mg/kg) in mice. -
Flammability and Explosibility
Benzene is a highly flammable liquid (NFPA rating = 3), and its vapors may travel a considerable distance to a source of ignition and "flash back." Vapor-air mixtures are explosive above the flash point. Carbon dioxide and dry chemical extinguishers should be used to fight benzene fires. -
Chemische Reaktivität
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. -
Industrielle Verwendung
Benzene (C6H6, CAS No. 71-43-2) is an aromatic hydrocarbon compound used extensively in the chemical industry as an intermediate in the manufacture of polymers and other products. It is also a common atmospheric contaminant and is present in motor vehicle exhaust emissions and cigarette smoke.
In 1990, it was discovered by the USA soft drinks industry that benzene could be produced at low levels in certain soft drinks containing a benzoate preservative and ascorbic acid. Since benzene is a known human carcinogen, its presence in food and beverages is clearly undesirable. -
Biochem/physiol Actions
Environmental carcinogen; hematoxin that is linked to increased incidence of leukemia in humans. -
Sicherheitsprofil
Confirmed human carcinogen producing myeloid leukemia, Hodgkin's dsease, and lymphomas by inhalation. Experimental carcinogenic, neoplastigenic, and tumorigenic data. A human poison by inhalation. An experimental poison by skin contact, intraperitoneal, intravenous, and possibly other routes. Moderately toxic by ingestion and subcutaneous routes. A severe eye and moderate sktn irritant. Human systemic effects by inhalation and ingestion: blood changes, increased body temperature. Experimental teratogenic and reproductive effects. Human mutation data reported. A narcotic. In industry, inhalation is the primary route of chronic benzene poisoning. Poisoning by skin contact has been reported. Recent (1 987) research indicates that effects are seen at less than 1 ppm. Exposures needed to be reduced to 0.1 ppm before no toxic effects were observed. Elimination is chiefly through the lungs. -
mögliche Exposition
Benzene is used as a constituent in motor fuels; as a solvent for fats; inks, oils, paints, plastics, and rubber, in the extraction of oils from seeds and nuts; in photogravure printing. It is also used as a chemical intermediate. By alkylation, chlorination, nitration, and sulfonation, chemicals, such as styrene, phenols, and malefic anhydride are produced. Benzene is also used in the manufacture of detergents, explosives, pharmaceuticals; in the manufacture of cyclohexane and ethylbenzene; and dye-stuffs. Increased concern for benzene as a significant environmental pollutant arises from public exposure to the presence of benzene in gasoline and the increased content in gasoline due to requirements for unleaded fuels for automobiles equipped with catalytic exhaust converters. -
Erste Hilfe
Eyes, skin, respiratory system, blood, central nervous system, bone marrow. Cancer site: leukemia. -
Carcinogenicity
Benzene is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. -
Environmental Fate
Benzene is released to air primarily by vaporization and combustion emissions associated with its use in gasoline. Other sources are vapors from its production and use in manufacturing other chemicals. In addition, benzene may be in industrial effluents discharged into water and accidental releases from gas and oil production, refining and distribution industries. Benzene released to soil will either evaporate very quickly or leach to groundwater. It can be biodegraded by soil and groundwater microbes. Benzene released to surface water should mostly evaporate within a few hours to a few days, depending on quantity, temperature, water turbulence, etc. Although benzene does not degrade by hydrolysis, it may be biodegraded by microbes. -
Lager
work with benzene should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Benzene should be used only in areas free of ignition sources. -
Versand/Shipping
UN1114 Benzene, Hazard Class: 3; Labels: 3— Flammable liquid -
läuterung methode
For most purposes, *benzene can be purified sufficiently by shaking with conc H2SO4 until free from thiophene, then with H2O, dilute NaOH and water, followed by drying (with P2O5, sodium, LiAlH4, CaH2, 4X Linde molecular sieve, or CaSO4, or by passage through a column of silica gel, and for a preliminary drying, CaCl2 is suitable), and distillation. A further purification step to remove thiophene, acetic acid and propionic acid, is crystallisation by partial freezing. The usual contaminants in dry thiophene-free *benzene are non-benzenoid hydrocarbons such as cyclohexane, methylcyclohexane, and heptanes, together with naphthenic hydrocarbons and traces of toluene. Carbonyl-containing impurities can be removed by percolation through a Celite column impregnated with 2,4-dinitrophenylhydrazine, phosphoric acid and H2O. (Prepared by dissolving 0.5g DNPH in 6mL of 85% H3PO4 by grinding together, then adding and mixing 4mL of distilled H2O and 10g Celite.) [Schwartz & Parker Anal Chem 33 1396 1961.] *Benzene has been freed from thiophene by refluxing with 10% (w/v) of Raney nickel for 15minutes, after which the nickel is removed by filtration or centrifugation. Dry *benzene is obtained by doubly distilling high purity *benzene from a solution containing the blue ketyl formed by the reaction of sodium-potassium alloy with a small amount of benzophenone. Thiophene has been removed from *benzene (absence of bluish-green coloration when 3mL of *benzene is shaken with a solution of 10mg of isatin in 10mL of conc H2SO4) by refluxing the *benzene (1.25L) for several hours with 40g HgO (freshly precipitated) dissolved in 40mL glacial acetic acid and 300mL of water. The precipitate is filtered off, the aqueous phase is removed and the *benzene is washed twice with H2O, dried and distilled. Alternatively, *benzene dried with CaCl2 has been shaken vigorously for 0.5hour with anhydrous AlCl3 (12g/L) at 25-35o, then decanted, washed with 10% NaOH, and water, dried and distilled. The process is repeated, giving thiophene-free *benzene. [Holmes & Beeman Ind Eng Chem 26 172 1934.] After shaking successively for about an hour with conc H2SO4, distilled water (twice), 6M NaOH, and distilled water (twice), *benzene is distilled through a 3-ft glass column to remove most of the water. Absolute EtOH is added and the *benzene-alcohol azeotrope is distilled. (This low-boiling distillation leaves any non-azeotrope-forming impurities behind.) The middle fraction is shaken with distilled water to remove EtOH, and again redistilled. Final slow and very careful fractional distillation from sodium, then LiAlH4 under N2, removed traces of water and peroxides. [Peebles et al. J Am Chem Soc 82 2780 1960.] *Benzene liquid and vapour are very TOXIC and HIGHLY FLAMMABLE, and all operations should be carried out in an efficient fume cupboard and in the absence of naked flames in the vicinity. [Beilstein 5 H 175, 5 I 95, 5 II 119, 5 III 469.] Rapid purification: To dry benzene, alumina, CaH2 or 4A molecular sieves (3% w/v) may be used (dry for 6hours). Then benzene is distilled, discarding the first 5% of distillate, and stored over molecular sieves (3A, 4A) or Na wire. -
Toxicity evaluation
Benzene enters the air, water, and soil as a result of industrial processes, emissions from burning coal and oil, tobacco smoke, gasoline exhaust, and gasoline leaks, and from natural sources, including volcanoes and forest fires. Benzene in the atmosphere chemically degrades in only a few days. Benzene released to soil or waterways is subject to volatilization, photooxidation, and biodegradation. Benzene has a short halflife in surface water because it is so volatile. -
Inkompatibilitäten
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, many fluorides and perchlorates, nitric acid. -
Filter für giftige stoffe
The acute initial threshold screening level (ITSL) for benzene (CAS # 71-43-2) is 30 μg/m3 based on a 24-hour averaging time. -
Waste disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Dilution with alcohol or acetone to minimize smoke is recommended. Bacterial degradation is also possible. -
Verordnung (Regulations)
Current USA and EU legislation does not set maximum limits for benzene in soft drinks. However, the FDA has adopted the Environmental Protection Agency (EPA) maximum contaminant level (MCL) for drinking water of 5 ppb as a quality standard for bottled water. ThisMCL has been used to evaluate the significance of benzene contamination in the soft drinks tested in surveys. The FSA has used the World Health Organization (WHO) guideline level for benzene in water of 10 mg kg-1 as a point of reference for its own survey results.
Benzene Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1of3
Downstream Produkte
- 1-Aziridinethanol
- 5-TERT-BUTYL-1H-INDOLE-2-CARBOXYLIC ACID ETHYL ESTER
- 1-Phenylnonan-1-on
- tert-Pentylbenzol
- 4-Benzoylbuttersaeure
- Isobutyrophenon
- Ethyl-3-(4-pyridyl)-3-oxopropionat
- 2-Methoxyphenylisocyanat
- 3-Acetamido-2,4,6-triiodbenzoesure
- Aminoethanolcarboxylate heptadecyl carbonate
- 3-THIOPHEN-2-YL-BENZALDEHYDE
- 2-(N-HEPTANOYL)THIOPHENE
- 2-Chlorphenylisocyanat
- Butyl-3-mercaptopropionat
- Phenylquecksilbernitrat
- 4-BROMO-7-CHLOROQUINOLINE
- 2,5-Dimethylhexin-2,5-diol
- 4-Methoxyphenylisocyanat
- 2-FLUOROPHENYL ISOCYANATE
- Cyclohexylphenylketon
- ISOCYANIC ACID
- Isomerase, Glucose
- Natrium-N-(6-chlorpyridazin-3-yl)sulfanilamidat
- 3-Nitrobenzoylchlorid
- Pesticide synergist agent
- 1,3-DIMETHYL-2-(2-THIENYL)IMIDAZOLIDINE
- 3-Brompropiononitril
- Octyl cyanoacetate
- Dichlorophenylborane
- 4-Fluorphenylisocyanat
1of8
Benzol Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(713)Suppliers
Region
-
Runte International Trade Limited
Telefon 19565631292<br/>19565631292
E-Mail lucky@sdruntechem.com
-
Telefon +1-3026880818<br/>+1-3026880818
E-Mail ARCTICEXPORTSINC@GMAIL.COM
-
Henan Fengda Chemical Co., Ltd
Telefon +86-371-86557731<br/>+86-13613820652
E-Mail info@fdachem.com
-
Henan Tianfu Chemical Co.,Ltd.
Telefon +86-0371-55170693<br/>+86-19937530512
E-Mail info@tianfuchem.com
-
Telefon +undefined-21-51877795
E-Mail ivan@atkchemical.com
-
Shanxi Naipu Import and Export Co.,Ltd
Telefon +86-13734021967<br/>+8613734021967
E-Mail kaia@neputrading.com
-
NINGBO INNO PHARMCHEM CO., LTD.
Telefon 13867897135
E-Mail sales@nbinno.com
-
Hubei Jusheng Technology Co.,Ltd.
Telefon 18871490254
E-Mail linda@hubeijusheng.com
-
Hubei xin bonus chemical co. LTD
Telefon 86-13657291602
E-Mail linda@hubeijusheng.com
-
Shandong chuangyingchemical Co., Ltd.
Telefon 18853181302
E-Mail sale@chuangyingchem.com
1of2
