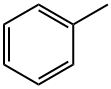
Toluol
Bezeichnung:Toluol
CAS-Nr108-88-3
Englisch Name:Toluene
CBNumberCB4233905
SummenformelC7H8
Molgewicht92.14
MOL-Datei108-88-3.mol
Synonyma
Toluol
Methylbenzol
Toluen
Phenylmethan
Toluol physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -93 °C (lit.) |
| Siedepunkt | 110-111 °C (lit.) |
| Dichte | 0.865 g/mL at 25 °C (lit.) |
| Dampfdichte | 3.2 (vs air) |
| Dampfdruck | 22 mm Hg ( 20 °C) |
| Brechungsindex | n |
| Flammpunkt | 40 °F |
| storage temp. | 0-6°C |
| pka | 40(at 25℃) |
| Aggregatzustand | Liquid |
| Farbe | Colorless |
| Wichte | 0.865~0.870(20/20℃)(Ph.Eur.) |
| Relative polarity | 0.099 |
| Geruch (Odor) | Aromatic, benzene-like odor detectable at 0.16 to 37 ppm (mean = 1.6 ppm) |
| Explosionsgrenze | 7% |
| Odor Threshold | 0.33ppm |
| Biologische Quelle | rabbit |
| Wasserlöslichkeit | 0.5 g/L (20 ºC) |
| Merck | 14,9529 |
| BRN | 635760 |
| Henry's Law Constant | 1.05 at 40 °C, 1.68 at 50 °C, 2.62 at 60 °C, 3.15 at 70 °C, 3.97 at 80 °C (headspace-GC, Vane et al., 2001) |
| Expositionsgrenzwerte | TLV-TWA 100 ppm (~375 mg/m3) (ACGIH, NIOSH, and MSHA), 200 ppm (~750 mg/ m3) OSHA; ceiling 300 ppm, peak 500 ppm/ 15 min (OSHA); STEL 150 ppm (ACGIH). |
| Dielectric constant | 2.4(20℃) |
| LogP | 2.73 at 20℃ |
| Oberflächenspannung | 27.76mN/m at 298.15K |
| CAS Datenbank | 108-88-3(CAS DataBase Reference) |
| IARC | 3 (Vol. 47, 71) 1999 |
| NIST chemische Informationen | Toluene(108-88-3) |
| EPA chemische Informationen | Toluene (108-88-3) |
| Kennzeichnung gefährlicher | F,Xn,T |
| R-Sätze: | 11-38-48/20-63-65-67-39/23/24/25-23/24/25 |
| S-Sätze: | 36/37-46-62-45-16-7 |
| RIDADR | UN 1294 3/PG 2 |
| OEB | A |
| OEL | TWA: 100 ppm (375 mg/m3), STEL: 150 ppm (560 mg/m3) |
| WGK Germany | 2 |
| RTECS-Nr. | XS5250000 |
| F | 3-10 |
| Selbstentzündungstemperatur | 480 °C |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29023000 |
| Giftige Stoffe Daten | 108-88-3(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 7.53 g/kg (Smyth) |
| IDLA | 500 ppm |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)



-
Alarmwort
Achtung
-
Gefahrenhinweise
H225:Flüssigkeit und Dampf leicht entzündbar.
H304:Kann bei Verschlucken und Eindringen in die Atemwege tödlich sein.
H315:Verursacht Hautreizungen.
H336:Kann Schläfrigkeit und Benommenheit verursachen.
H373:Kann die Organe schädigen bei längerer oder wiederholter Exposition.
H412:Schädlich für Wasserorganismen, mit langfristiger Wirkung.
-
Sicherheit
P201:Vor Gebrauch besondere Anweisungen einholen.
P210:Von Hitze, heißen Oberflächen, Funken, offenen Flammen und anderen Zündquellenarten fernhalten. Nicht rauchen.
P273:Freisetzung in die Umwelt vermeiden.
P302+P352:BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen.
P308+P313:BEI Exposition oder falls betroffen: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
Toluene Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
FARBLOSE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH. -
PHYSIKALISCHE GEFAHREN
Die Dämpfe mischen sich leicht mit Luft. Bildung explosionsfähiger Gemische. Fließen, Schütten o.ä. kann zu elektrostatischer Aufladung führen. -
CHEMISCHE GEFAHREN
Reagiert sehr heftig mit starken Oxidationsmitteln. Feuer- und Explosionsgefahr. -
ARBEITSPLATZGRENZWERTE
TLV: 50 ppm (als TWA); Hautresorption; Krebskategorie A4 (nicht klassifizierbar als krebserzeugend für den Menschen); BEI vorhanden; (ACGIH 2005).
MAK: 50 ppm 190 mg/m? Spitzenbegrenzung: überschreitungsfaktor II(4); Hautresorption; Schwangerschaft: Gruppe C; (DFG 2005).
EG Arbeitsplatz-Richtgrenzwerte: 192 mg/m? 50 ppm (als TWA); 100 ppm (als STEL); Hautresorption; (EG 2006)
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation, über die Haut und durch Verschlucken. -
INHALATIONSGEFAHREN
Beim Verdampfen bei 20 °C kann schnell eine gesundheitsschädliche Kontamination der Luft eintreten. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz reizt die Augen und die Atmungsorgane. Möglich sind Auswirkungen auf das Zentralnervensystem. Verschlucken kann zur Aufnahme in der Lunge führen; Gefahr der Aspirationspneumonie. Exposition gegenüber hohen Konzentrationen kann zu Herzrhythmusstörungen und -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Die Flüssigkeit entfettet die Haut. Möglich sind Auswirkungen auf das Zentralnervensystem. Exposition gegenüber der Substanz kann durch Lärmeinwirkung verursachte Hörschäden verstärken. Tierversuche zeigen, dass die Substanz möglicherweise fruchtbarkeitsschädigend oder entwicklungsschädigend wirken kann. -
R-Sätze Betriebsanweisung:
R11:Leichtentzündlich.
R38:Reizt die Haut.
R48/20:Gesundheitsschädlich: Gefahr ernster Gesundheitsschäden bei längerer Exposition durch Einatmen.
R63:Kann das Kind im Mutterleib möglicherweise schädigen.
R65:Gesundheitsschädlich: kann beim Verschlucken Lungenschäden verursachen.
R67:Dämpfe können Schläfrigkeit und Benommenheit verursachen.
R39/23/24/25:Giftig: ernste Gefahr irreversiblen Schadens durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R23/24/25:Giftig beim Einatmen, Verschlucken und Berührung mit der Haut. -
S-Sätze Betriebsanweisung:
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S46:Bei Verschlucken sofort ärztlichen Rat einholen und Verpackung oder Etikett vorzeigen.
S62:Bei Verschlucken kein Erbrechen herbeiführen. Sofort ärztlichen Rat einholen und Verpackung oder dieses Etikett vorzeigen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen). -
Aussehen Eigenschaften
C7H8; Methylbenzol, Toluen, Benzylwasserstoff. Farblose Flüssigkeit mit charakteristischem Geruch. -
Gefahren für Mensch und Umwelt
Gesundheitsschädlich beim Einatmen. Reizt die Augen, Atmungsorgane und die Haut. Wird über die Haut aufgenommen. Aufnahme grösserer Mengen führt zu Rausch, Krämpfen bis zur Bewusstlosigkeit.
Nicht stark erhitzen. Nicht mit Halogen-Halogenverbindungen, Stickstoffoxiden, Salpetersäure, Uranhexafluorid, organischen Nitroverbindungen, Oxidationsmittel und Schwefel in der Hitze in Berührung bringen.
Leichtentzündlich.
LD50 (oral, Ratte): 5000 mg/kg -
Schutzmaßnahmen und Verhaltensregeln
Schutzhandschuhe als kurzzeitiger Spritzschutz. -
Verhalten im Gefahrfall
Persönliche Maßnahmen: Dämpfe nicht einatmen.
Mit flüssigkeitsbindendem Material aufnehmen. Der Entsorgung zuführen. Nachreinigen.
Kohlendioxid, Pulver, Schaum.
Mit Luft Bildung explosionsfähiger Gemische. Dämpfe schwerer als Luft. -
Erste Hilfe
Nach Hautkontakt: Mit reichlich Wasser abwaschen.
Nach Augenkontakt: Mit reichlich Wasser bei geöffnetem Lidspalt mindestens 15 Minuten ausspülen. Sofort Augenarzt hinzuziehen.
Nach Einatmen: Frischluft. Arzt hinzuziehen.
Nach Verschlucken: Wasser trinken. Paraffinöl (3 ml/kg) und Natriumsulfat (1 Esslöffel auf 250 ml Wasser) geben. Sofort Arzt hinzuziehen.
Nach Kleidungskontakt: Kontaminierte Kleidung sofort entfernen.
Ersthelfer: siehe gesonderten Anschlag -
Sachgerechte Entsorgung
Als halogenfreie, organische Lösemittelabfälle. -
Beschreibung
Toluene is a clear, colourless liquid with a sweet, benzene-like odour. Toluene occurs naturally in crude oil and in the toluene tree. It is also produced in the process of making gasoline and other fuels from crude oil and making coke from coal. Toluene is used in making paints, paint thinners, fingernail polish, lacquers, adhesives, and rubber and in some printing and leather tanning processes. Toluene is also used in the production of polymers used to make nylon, plastic soda bottles, and polyurethanes and for pharmaceuticals, dyes, cosmetic nail products, and the synthesis of organic chemicals.
Toluene has been reported as the most commonly abused hydrocarbon solvent, primarily through ‘glue sniffing’. The common possibilities of exposure to high levels of toluene include indoor air from the use of household products such as paints, paint thinners, adhesives, synthetic fragrances, and many other sources. -
Chemische Eigenschaften
Toluene is a clear, colorless, flammable liquid with a sweet/pungent odor. It is extensively used as a solvent in different industries, i.e., rubber chemical manufacturing, drugs and pharmaceuticals, thinner for inks, paints dyes, and perfume manufacturing. It is a natural constituent of crude oil and is produced from petroleum refi ning and coke-oven operations. Toluene occurs naturally as a component of crude oil and occurs in petroleum refi ning and coke oven operations. Occupational workers associated with several kinds of activities, such as manufacturing of dyes, printing inks, painting automobile mechanics, gasoline manufacturers, shippers, and retailers, adhesives and coatings manufacturers and applicators, audio-equipment product workers, chemical industry workers, coke-oven workers, fabric manufacturers (fabric coating), sites of hazardous wastes, linoleum manufacturers, in pharmaceutical manufacturing, printing works, shoe manufacturing industry, become exposed to toluene. -
Chemische Eigenschaften
Also known as methyl benezne or phenyl methane, C6H5CH3 is a flammable, toxic, colorless liquid. Insoluble in water and soluble in alcohol and ether, it is used in explosives,high-octane gasoline,and organic synthesis. -
Chemische Eigenschaften
Toluene is a clear, colorless, noncorrosive liquid with a sweet, pungent, benzene-like odor. The Odor Threshold in air is variously given as 0.17 ppm, 2.9 ppm (NJ) and 8 ppm (EPA). The Odor Threshold in water is 0.04-1.0 mg/L -
Physikalische Eigenschaften
Colorless, clear, flammable liquid with a pleasant, sweet or paint-like odor similar to benzene. At 40 °C, the lowest concentration at which an odor was detected were 960 μg/L. Similarly at 25 °C, the lowest concentration at which a taste was detected was 960 μg/L (Young et al., 1996). Experimentally determined detection and recognition odor threshold concentrations were 600 μg/m3 (160 ppbv) and 7.0 mg/m3 (1.9 ppmv), respectively (Hellman and Small, 1974). Leonardos et al. (1969) reported higher odor threshold concentrations for toluene derived from coke (4.68 ppmv) and petroleum (2.14 ppmv). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 24 and 140 μg/L, respectively (Alexander et al., 1982). An odor threshold concentration of 330 ppbv was determined by a triangular odor bag method (Nagata and Takeuchi, 1990). Cometto-Mu?iz and Cain (1994) reported an average nasal pungency threshold concentration of 29,574 ppmv. -
History
Toluene is a clear, flammable, aromatic hydrocarbon liquid with a smell similar to benzene. It is also called methylbenzene, indicating that a methyl group has been added to one of benzene’s carbon atoms. Toluene was first isolated by Pierre-Joseph Pelletier (1788–1842) and Philippe Walter (1810–1847) in 1837. The name toluene comes from the South American tree Toluifera balsamum. Henri-Etienne Sainte-Claire Deville (1818–1881) isolated toluene from the tree’s gum, Tolu balsam, in 1841.
The main source of toluene is from the catalytic reforming of naphthas during petroleum processing. During this process cycloalkanes are dehydrated, forming aromatics such as toluene and xylene along with hydrogen. Toluene can also be obtained from the pyrolysis of gasoline. It is a by-product when styrene is produced and can also be produced from coal tar, which was its main source in the first half of the 20th century. -
Verwenden
Toluene is derived from coal tar as well aspetroleum. It occurs in gasoline and manypetroleum solvents. Toluene is used to producetrinitrotoluene (TNT), toluene diisocyanate,and benzene; as an ingredient fordyes, drugs, and detergents; and as an industrialsolvent for rubbers, paints, coatings, andoils. -
Verwenden
In manufacture of benzoic acid, benzaldehyde, explosives, dyes, and many other organic Compounds; as a solvent for paints, lacquers, gums, resins; thinner for inks, perfumes, dyes; in the extraction of various principles from plants; as gasoline additive. -
Verwenden
Toluene has numerous applications in the chemical and petroleum industry, with approximately 6 million tons used annually in the United States and 16 million tons used globally. The major use of toluene is as an octane booster in gasoline. Toluene has an octane rating of 114. Toluene is one of the four principal aromatic compounds, along with benzene, xylene, and ethylbenzene, that are produced during refining to enhance gasoline's performance. Collectively, these four compounds are abbreviated as BTEX. BTEX is a major component of gasoline, forming about 18% by weight of a typical blend. Although the proportion of the aromatics is varied to produce different blends to meet geographic and seasonal requirements, toluene is one of the major components. A typical gasoline contains approximately 5% toluene by weight.
Toluene is a primary feedstock used to produce various organic compounds. It is usedto produce diisocyanates. Isocyanates contain the functional group ?N = C = O, and diisocyanatescontain two of these. The two main diisocyanates are toluene 2,4-diisocyanate andtoluene 2,6-diisocyanate. The production of diisocyanates in North America is close to a billionpounds annually. More than 90% of toluene diisocyanate production is used for makingpolyurethanes foams. The latter are used as flexible fill in furniture, bedding, and cushions.In rigid form it is used for insulation, hard shell coatings, building materials, auto parts, androller skate wheels. -
Definition
ChEBI: The simplest member of the class toluenes consisting of a benzene core which bears a single methyl substituent. -
Vorbereitung Methode
Benzene is produced from toluene through a process called hydrodealkylation. In thisprocess, toluene reacts with hydrogen in the presence of a chromium, platinum, or molybdenumcatalysts at temperatures of several hundred degrees Celsius and pressures of about50 atmospheres: C6H5CH3 + H2 → C6H6 + CH4. Toluene can also be used to producephenol, (C6H5OH), benzoic acid (C6H5COOH), and benzaldehyde (C6H5CHO). Nitratedforms of toluene produce explosive compounds; the most common of these is TNT (SeeTrinitrotoluene). -
synthetische
Toluene is the starting material for the production of tolylene diisocyanate (TDI),the process may be varied to give products of differing isomer contents. The nitration of toluene (with a nitrating mixture containing 20% nitric acid, 60% sulphuric acid and 20% water at 30-45°C) gives a mixture of 2-nitrotoluene (about 60%) and 4- nitrotoluene (40%). If this mixture is nitrated further (with a mixture of 35% nitric acid and 65% sulphuric acid at 65-80°C) without separation, the product is a mixture of2,4-dinitrotoluene (about 80%) and 2,6-dinitrotoluene (20%). If, on the other hand, the mixed mononitrates are separated (by distillation), then further nitration of the 2-nitrotoluene yields a mixture of 2,4-dinitrotoluene (about 65%) and 2,6-dinitrotoluene (35%) whilst further nitration of the 4-nitrotoluene gives only 2,4-dinitrotoluene. -
Synthesis Reference(s)
Canadian Journal of Chemistry, 55, p. 3755, 1977 DOI: 10.1139/v77-529
Chemistry Letters, 11, p. 1707, 1982
Tetrahedron Letters, 17, p. 2689, 1976 -
Allgemeine Beschreibung
A clear colorless liquid with a characteristic aromatic odor. Flash point 40°F. Less dense than water (7.2 lb / gal) and insoluble in water. Hence floats on water. Vapors heavier than air. May be toxic by inhalation, ingestion or skin contact. Used in aviation and automotive fuels, as a solvent, and to make other chemicals. -
Air & Water Reaktionen
Highly flammable. Insoluble in water. -
Reaktivität anzeigen
Toluene reacts vigorously with allyl chloride or other alkyl halides even at minus 70° C in the presence of ethyl aluminum dichloride or ethyl aluminum sesquichloride. Explosions have been reported [NFPA 491M 1991]. Incompatible with strong oxidizing agents. When added to a tank of sulfur dichloride, the tank over pressurized and ruptured in a reaction thought to be catalyzed by iron or iron(III) chloride [Chem. Eng. News, 1988, 66(32), 2]. -
Hazard
Flammable, dangerous fire risk. Explosive limits in air 1.27–7%. Toxic by ingestion, inhalation, and skin absorption. Visual impairment, female reproductive effects, and pregnancy loss. Questionable carcinogen. -
Health Hazard
Exposures to toluene cause adverse health effects to animals and humans. The symptoms of toxicity and poisoning include, but are not limited to, mild irritation to the skin, headache, nausea, and effects on the CNS. Prolonged exposure to high concentrations of toluene causes disturbances in vision, dizziness, nausea, CNS depression, paresthesia, and sudden collapse. The acute oral LD50 value of toluene in laboratory rats has been reported as 636–7300 mg/kg. Exposure to toluene has been reported to cause rapid and severe corneal damage and conjunctiva infl ammation. The acute dermal LD50 in rabbits was found to be between 1200 and 1400 mg/kg. -
Health Hazard
The acute toxicity of toluene is low. Toluene may cause eye, skin, and respiratory tract irritation. Short-term exposure to high concentrations of toluene (e.g., 600 ppm) may produce fatigue, dizziness, headaches, loss of coordination, nausea, and stupor; 10,000 ppm may cause death from respiratory failure. Ingestion of toluene may cause nausea and vomiting and central nervous system depression. Contact of liquid toluene with the eyes causes temporary irritation. Toluene is a skin irritant and may cause redness and pain when trapped beneath clothing or shoes; prolonged or repeated contact with toluene may result in dry and cracked skin. Because of its odor and irritant effects, toluene is regarded as having good warning properties.
The chronic effects of exposure to toluene are much less severe than those of benzene. No carcinogenic effects were reported in animal studies. Equivocal results were obtained in studies to determine developmental effects in animals. Toluene was not observed to be mutagenic in standard studies. -
Health Hazard
Vapors irritate eyes and upper respiratory tract; cause dizziness, headache, anesthesia, respiratory arrest. Liquid irritates eyes and causes drying of skin. If aspirated, causes coughing, gagging, distress, and rapidly developing pulmonary edema. If ingested causes vomiting, griping, diarrhea, depressed respiration. -
Health Hazard
The acute toxicity of toluene is similar to thatof benzene. The exposure routes are inhalation,ingestion, and absorption through theskin; and the organs affected from its exposureare the central nervous system, liver,kidneys, and skin. At high concentrations it is a narcotic. In humans, acute exposure canproduce excitement, euphoria, hallucination,distorted perceptions, confusion, headache,and dizziness. Such effects may be perceptibleat an exposure level of 200 ppm in air.Higher concentrations can produce depression,drowsiness, and stupor. Inhalation of10,000 ppm may cause death to humans fromrespiratory failure.
Toluene is metabolized to benzoic acidand finally, to hippuric acid and benzoylglucuronide.The latter two are excreted in urinealong with small amounts of cresols, formedby direct hydroxylation of toluene. Chronicexposure may cause some accumulation oftoluene in fatty tissues, which may be eliminatedover a period of time. The chroniceffects of toluene are much less severe to benzene.It is not known to cause bone marrowdepression or anemia. Animal tests showedno carcinogenic effects. -
Brandgefahr
Behavior in Fire: Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. -
Brandgefahr
Toluene is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Toluene vapor forms explosive mixtures with air at concentrations of 1.4 to 6.7% (by volume). Hazardous gases produced in fire include carbon monoxide and carbon dioxide. Carbon dioxide and dry chemical extinguishers should be used to fight toluene fires. -
Flammability and Explosibility
Toluene is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Toluene vapor forms explosive mixtures with air at concentrations of 1.4 to 6.7% (by volume). Hazardous gases produced in fire include carbon monoxide and carbon dioxide. Carbon dioxide and dry chemical extinguishers should be used to fight toluene fires. -
Chemische Reaktivität
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. -
Sicherheitsprofil
Poison by intraperitoneal route. Moderately toxic by intravenous and subcutaneous routes. Mddly toxic by inhalation. An experimental teratogen. Human systemic effects by inhalation: CNS recordtng changes, hallucinations or distorted perceptions, motor activity changes, antipsychotic, psychophysiological test changes, and bone marrow changes. Experimental reproductive effects. Mutation data reported. A human eye irritant. An experimental skin and severe eye irritant. Toluene is derived from coal tar, and commercial grades usually contain small amounts of benzene as an impurity. Inhalation of 200 ppm of toluene for 8 hours may cause impairment of coordtnation and reaction time; with higher concentrations (up to 800 ppm) these effects are increased and are observed in a shorter time. In the few cases of acute toluene poisoning reported, the effect has been that of a narcotic, the workman passing through a stage of intoxication into one of coma. Recovery following removal from exposure has been the rule. An occasional report of chronic poisoning describes an anemia and leukopenia, with biopsy showing a bone marrow hypoplasia. These effects, however, are less common in people working with toluene, and they are not as severe. At 200-500 ppm, headache, nausea, eye irritation, loss of appetite, a bad taste, lassitude, impairment of coordination and reaction time are reported, but are not usually accompanied by any laboratory or physical findings of significance. With higher concentrations, the above complaints are increased and in addition, anemia, leukopenia, and enlarged liver may be found in rare cases. A common air contaminant, emitted from modern building materials - (CENEAR 69,22,91). Used in production of drugs of abuse. Flammable liquid. A very dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosive in the form of vapor when exposed to heat or flame. Explosive reaction with 1,3-dtchloro-5,5-dimethyl-2,4- imidazolididione, dinitrogen tetraoxide, concentrated nitric acid, H2SO4 + HNO3, N2O4, AgClO4, BrF3, UF6, sulfur dichloride. Forms an explosive mixture with tetranitromethane. Can react vigorously with oxidtzing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. -
mögliche Exposition
Toluene is used as an industrial chemical, chemical intermediate; solvent, and emulsifier; may be encountered in the manufacture of benzene. It is also used as a chemical feed for toluene diisocyanate, phenol, benzyl and benzoyl derivatives; benzoic acid; toluene sulfonates; nitrotoluenes, vinyltoluene, and saccharin; as a solvent for paints and coatings; or as a component of automobile and aviation fuels. -
Carcinogenicity
The IARC has determined that there is evidence for the lack of carcinogenicity of toluene in experimental animals and that there is inadequate evidence for carcinogenicity in humans. Results of in vitro assays generally indicate that toluene is not genotoxic. Reports of increased incidences of sister chromatid exchanges and chromatid breaks in exposed workers are confounded by concurrent exposure to other organic chemicals. -
Source
Detected in distilled water-soluble fractions of 87 octane gasoline (25.9 mg/L), 94 octane gasoline (86.9 mg/L), Gasohol (60.8 mg/L), No. 2 fuel oil (1.54 mg/L), jet fuel A (1.05 mg/L), diesel fuel (0.86 mg/L), military jet fuel (JP-4 (32.0 mg/L) (Potter, 1996), new motor oil (16.3 to 16.9 8 μg/L), and used motor oil (781–814 μg/L) (Chen et al., 1994). The average volume percent and estimated mole fraction in American Petroleum Institute PS-6 gasoline are 3.519 and 0.04392, respectively (Poulsen et al., 1992). Schauer et al. (1999) reported toluene in a diesel-powered medium-duty truck exhaust at an emission rate of 3,980 μg/km. Diesel fuel obtained from a service station in Schlieren, Switzerland contained toluene at an estimated concentration of 374 mg/L (Schluep et al., 2001).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for 24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method 602. Average toluene concentrations reported in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were 23.676, 1.065, and 0.552 mg/L, respectively. When the authors analyzed the aqueous-phase via U.S. EPA approved test method 610, average toluene concentrations in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were lower, i.e., 12.969, 0.448, and 0.030 mg/L, respectively.
Kaplan et al. (1996) determined toluene concentrations in four different grades of gasolines. Average toluene concentrations were 32.6 g/L in regular unleaded gasoline, 28.7 g/L in leaded gasoline, 36.7 g/L in unleaded plus gasoline, and 40.9 g/L in Super unleaded gasoline. Harley et al. (2000) analyzed the headspace vapors of three grades of unleaded gasoline where ethanol was added to replace methyl tert-butyl ether. The gasoline vapor concentrations of toluene in the headspace were 1.9 wt % for regular grade, 1.8 wt % for mid-grade, and 2.0 wt % for premium grade.
In 7 coal tar samples, toluene concentrations ranged from ND to 7,000 ppm (EPRI, 1990). Detected in 1-yr aged coal tar film and bulk coal tar at concentrations of <75 and 220 mg/kg, respectively (Nelson et al., 1996). A high-temperature coal tar contained toluene at an average concentration of 0.25 wt % (McNeil, 1983).
Identified as one of 140 volatile constituents in used soybean oils collected from a processing plant that fried various beef, chicken, and veal products (Takeoka et al., 1996). Schauer et al. (2001) measured organic compound emission rates for volatile organic compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission rate of toluene was 158 mg/kg of pine burned. Emission rates of toluene were not measured during the combustion of oak and eucalyptus.
Reported as an impurity (≤ 0.8 wt %) in 98.5 wt % benzyl mercpatan (Chevron Phillips, April 2005).
Drinking water standard (final): MCLG: 1 mg/L; MCL: 1 mg/L. In addition, a DWEL of 7 μg/L was recommended (U.S. EPA, 2000). -
Environmental Fate
Biological. Toluene can undergo two types of microbial attack. The first type proceeds via immediate hydroxylation of the benzene ring, followed by ring cleavage. The second type of attack proceeds via oxidation of the methyl group followed by hydroxylation and ring cleavage (Fewson, 1981). A mutant of Pseudomonas putida oxidized toluene to (+)-cis-2,3-dihydroxy-1- methylcyclohexa-1,4-diene (Dagley, 1972). Claus and Waker (1964) reported that Pseudomonas sp. and an Achromobacter sp. oxidized toluene to 3-methylcatechol. Other metabolites identified in the microbial degradation of toluene include cis-2,3-dihydroxy-2,3-dihydrotoluene, 3- methylcatechol, benzyl alcohol, benzaldehyde, benzoic acid, catechol (quoted, Verschueren, 1983), and 1-hydroxy-2-naphthoic acid (Claus and Walker, 1964). In a methanogenic aquifer material, toluene degraded completely to carbon dioxide (Wilson et al., 1986). In activated sludge, 26.3% of the applied toluene mineralized to carbon dioxide after 5 d (Freitag et al., 1985). Based on a first-order degradation rate constant of 0.07/yr, the half-life of toluene is 39 d (Zoeteman et al., 1981).
Photolytic. Cox et al. (1980) reported a rate constant of 7.2 x 10-12 cm3/molecule?sec for the reaction of gaseous toluene with OH radicals based on a value of 8 x 10-12 cm3/molecule?sec for the reaction of ethylene with OH radicals.
Surface Water. Mackay and Wolkoff (1973) estimated an evaporation half-life of 30.6 min from a surface water body that is 25 °C and 1 m deep.
Groundwater. Nielsen et al. (1996) studied the degradation of toluene in a shallow, glaciofluvial, unconfined sandy aquifer in Jutland, Denmark. As part of the in situ microcosm study, a cylinder that was open at the bottom and screened at the top was installed through a cased borehole approximately 5 m below grade. Five liters of water was aerated with atmospheric air to ensure aerobic conditions were maintained. Groundwater was analyzed weekly for approximately 3 months to determine toluene concentrations with time. The experimentally determined firstorder biodegradation rate constant and corresponding half-life following a 5-d lag phase were 0.4/d and 1.73 d, respectively.
Photolytic. Synthetic air containing gaseous nitrous acid and toluene exposed to artificial sunlight (λ = 300–450 nm) yielded methyl nitrate, peroxyacetal nitrate, and a nitro aromatic compound tentatively identified as a nitrophenol or nitrocresol (Cox et al., 1980). A n-hexane solution containing toluene and spread as a thin film (4 mm) on cold water (10 °C) was irradiated by a mercury medium pressure lamp. In 3 h, 26% of the toluene photooxidized into benzaldehyde, benzyl alcohol, benzoic acid, and m-cresol (Moza and Feicht, 1989). Methane and ethane were reported as products of the gas-phase photolysis of toluene at 2537 ? (Calvert and Pitts, 1966).
Chemical/Physical. Products identified from the reaction of toluene with nitric oxide and OH radicals include benzaldehyde, benzyl alcohol, 3-nitrotoluene, p-methylbenzoquinone, and o-, m-, and p-cresol (Kenley et al., 1978). Gaseous toluene reacted with nitrate radicals in purified air forming the following products: benzaldehyde, benzyl alcohol, benzyl nitrate, and 2-, 3-, and 4- nitro-toluene (Chiodini et al., 1993). Under atmospheric conditions, the gas-phase reaction with OH radicals and nitrogen oxides resulted in the formation of benzaldehyde, benzyl nitrate, 3- nitrotoluene, and o-, m-, and p-cresol (Finlayson-Pitts and Pitts, 1986; Atkinson, 1990). -
Solubility in organics
Soluble in acetone, carbon disulfide, and ligroin; miscible with acetic acid, ethanol, benzene, ether, chloroform (U.S. EPA, 1985), and other organic solvents including xylenes, toluene, and ethylbenzene. -
Lager
toluene should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers. -
Versand/Shipping
UN1294 Toluene, Hazard Class: 3; Labels: 3-Flammable liquid. -
läuterung methode
Dry toluene with CaCl2, CaH2 or CaSO4, and dry further by standing with sodium, P2O5 or CaH2. It can be fractionally distilled from sodium or P2O5. Unless specially purified, toluene is likely to be contaminated with methylthiophenes and other sulfur-containing impurities. These can be removed by shaking with conc H2SO4, but the temperature must be kept below 30o if sulfonation of toluene is to be avoided. A typical procedure consists of shaking toluene twice with cold conc H2SO4 (100mL of acid per L), once with water, once with aqueous 5% NaHCO3 or NaOH, again with H2O, then drying successively with CaSO4 and P2O5, with final distillation from P2O5 or over LiAlH4 after refluxing for 30minutes. Alternatively, the treatment with NaHCO3 can be replaced by boiling under reflux with 1% sodium amalgam. Sulfur compounds can also be removed by prolonged shaking of the toluene with mercury, or by two distillations from AlCl3, the distillate then being washed with water, dried with K2CO3 and stored with sodium wire. Other purification procedures include refluxing and distillation of sodium dried toluene from diphenylpicrylhydrazyl, and from SnCl2 (to ensure freedom from peroxides). It has also been co-distilled with 10% by volume of ethyl methyl ketone, and again fractionally distilled. [Brown & Pearsall J Am Chem Soc 74 191 1952.] For removal of carbonyl impurities see *benzene. Toluene has been purified by distillation under nitrogen in the presence of sodium benzophenone ketyl. Toluene has also been dried with MgSO4, after the sulfur impurities have been removed, and then fractionally distilled from P2O5 and stored in the dark [Tabushi et al. J Am Chem Soc 107 4465 1985]. Toluene can be purified by passage through a tightly packed column of Fuller's earth. Rapid purification: Alumina, CaH2 and 4A molecular sieves (3% w/v) may be used to dry toluene (6hours stirring and standing). Then the toluene is distilled, discarding the first 5% of distillate, and is stored over molecular sieves (3A, 4A) or Na wire. [Beilstein 5 H 280, 5 I 144, 5 II 209, 5 III 651, 5 IV 766.] -
Toxicity evaluation
The mechanism of toxicity is suspected to be similar to other solvents that rapidly induce anesthesia-like effects, i.e., a ‘nonspecific narcosis’ due to disruption (solvation) of the integrity of the cellular membranes of the central nervous system (CNS). The effect is similar to the ‘high’ experienced upon exposure to other hydrocarbon solvents.
As seen with exposure to other hydrocarbon solvents, upon inhalation, toluene is moderately toxic and may cause irritation of the respiratory tract and narcosis. Toluene appears to produce reversible effects on the liver, renal, and nervous systems. The nervous system appears to be the most sensitive to the effects of toluene. High-level toluene exposures produced incoordination, ataxia, unconsciousness, and, eventually, death. Lower level acute exposures in man produce dizziness, exhilaration, and confusion. Although the actual biochemical mechanism of toxicity has not been discerned, the narcotic effects seen are most likely related to its physical solvent properties. -
Inkompatibilitäten
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Violent reaction with mixtures of nitric and sulfuric acid. -
Filter für giftige stoffe
The initial threshold screening level (ITSL) for toluene is 5,000 μg/m3 based on a 24-hour averaging time. -
Waste disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Toluene Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1of3
Downstream Produkte
- Chloroprene-phenolic adhesive 801
- Hexahydrocyclopenta[c]pyrrol-2(1H)-aminmonohydrochlorid
- QUINOLINE-2-CARBONYL CHLORIDE
- 2-Thienyl isocyanate
- 3',6'-Dichlorspiro(phthalid-3,9'-xanthen)
- (R)-Glycidyl butyrate
- 1-(2-CHLOROBENZYL)PIPERAZINE
- 5-BROMOPYRIDINE-2-CARBONYL CHLORIDE
- 2-(2-Hexyloxyethoxy)ethanol
- Asphalt antifouling paint L40-32
- Methyltetrahydro-4-oxo-3-thenoat
- (Methoxymethyl)triphenylphosphoniumchlorid
- 2-Methylpropylendiamin
- N-(Chlormethyl)phthalimid
- TRIS(NONYLPHENYL) PHOSPHITE
- Bis(benzonitril)palladiumchlorid
- 1,3-DIMETHYL-2-(2-FURYL)IMIDAZOLIDINE
- 1-Butyl-3-methylimidazolium bromide
- Antimontriacetat
- 1-(3-CHLOROBENZYL)PIPERAZINE
- 3-Thenoylchlorid
- 2-AMINO-4-METHOXYPYRIMIDINE
- 3-Cyanophenyl isocyanate
- Dichloro[(1,2,5,6-?)-cycloocta-1,5-dien]palladium
- 6-FLUORO-4-HYDROXY-2-(TRIFLUOROMETHYL)QUINOLINE
- 4-Amino-6-chlorpyrimidin-5-carbaldehyd
- Trimethylamin, N-oxid
- (11AR)-(+)-10,11,12,13-TETRAHYDRODIINDENO[7,1-DE:1',7'-FG][1,3,2]DIOXAPHOSPHOCIN-5-DIMETHYLAMINE
- 3-Hydroxy-alpha-((methylamino)-methyl)benzolethanol-hydro-chlorid, (R)
- Trimethylsilyl-N-trimethylsilyl-acetamidat
1of8
Toluol Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(1)Suppliers
-
Yurui (Shanghai) Chemical Co., Ltd.
Telefon +86-021-50456736<br/>+8613761615711
E-Mail yuki@riyngroup.com
