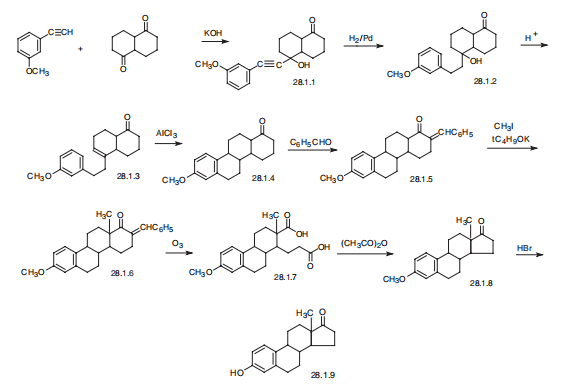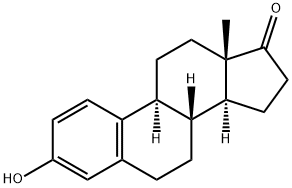
Estron
Bezeichnung:Estron
CAS-Nr53-16-7
Englisch Name:Estrone
CBNumberCB5741416
SummenformelC18H22O2
Molgewicht270.37
MOL-Datei53-16-7.mol
Synonyma
Estron
Estron physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 258-260 °C(lit.) |
| alpha | 158 º (c=1, dioxane) |
| Siedepunkt | 353.48°C (rough estimate) |
| Dichte | 1.2360 |
| Brechungsindex | 165 ° (C=1, Dioxane) |
| Flammpunkt | 9℃ |
| storage temp. | room temp |
| Löslichkeit | Chloroform (Slightly), Dioxane (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| pka | pKa 10.77±0.02(H2O)(Approximate) |
| Aggregatzustand | Crystalline Powder or Crystals |
| Farbe | White to almost white |
| Wasserlöslichkeit | 0.03 g/L |
| Merck | 3708 |
| BRN | 1915077 |
| InChIKey | DNXHEGUUPJUMQT-CBZIJGRNSA-N |
| CAS Datenbank | 53-16-7(CAS DataBase Reference) |
| NIST chemische Informationen | 3-Hydroxyestra-1,3,5(10)-trien-17-one(53-16-7) |
| EPA chemische Informationen | Estrone (53-16-7) |
| Kennzeichnung gefährlicher | T,F |
| R-Sätze: | 45-60-61-64-40-63-39/23/24/25-23/24/25-11 |
| S-Sätze: | 53-45-36/37-16 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS-Nr. | KG8575000 |
| HS Code | 29335995 |
| Giftige Stoffe Daten | 53-16-7(Hazardous Substances Data) |