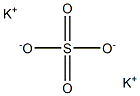
Kaliumsulfat
Bezeichnung:Kaliumsulfat
CAS-Nr7778-80-5
Englisch Name:Potassium sulfate
CBNumberCB9854321
SummenformelK2O4S
Molgewicht174.2592
MOL-Datei7778-80-5.mol
Synonyma
Kaliumsulfat
Dikaliumsulfat
Kaliumsulfat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 1067°C |
| Siedepunkt | 1689°C |
| chüttdichte | 800kg/m3 |
| Dichte | 2.66 |
| Flammpunkt | 1689°C |
| storage temp. | Inert atmosphere,Room Temperature |
| Löslichkeit | H2O: 0.5 M at 20 °C, clear, colorless |
| Aggregatzustand | Very Fine Crystals or Powder |
| Farbe | White |
| Wichte | 2.662 |
| Geruch (Odor) | Odorless |
| Säure-Base-Indikators(pH-Indikatoren) | ~7 |
| PH | 5.5-7.5 (25℃, 0.5M in H2O) |
| Biologische Quelle | synthetic |
| Wasserlöslichkeit | 110 g/L (20 ºC) |
| maximale Wellenlänge (λmax) | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,7674 |
| crystal system | Nogata |
| S-Sätze: | 22-24/25 |
| WGK Germany | 1 |
| RTECS-Nr. | TT5900000 |
| TSCA | Yes |
| HS Code | 31043000 |
| Giftige Stoffe Daten | 7778-80-5(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 6600 mg/kg |


