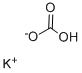
Kaliumhydrogencarbonat
Bezeichnung:Kaliumhydrogencarbonat
CAS-Nr298-14-6
Englisch Name:Potassium bicarbonate
CBNumberCB3260704
SummenformelCHKO3
Molgewicht100.12
MOL-Datei298-14-6.mol
Synonyma
Kaliumhydrogencarbonat
Kaliumhydrogencarbonat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 292 °C |
| Dichte | 2,17 g/cm3 |
| storage temp. | Store at +15°C to +25°C. |
| Löslichkeit | H2O: 1 M at 20 °C, clear, colorless |
| Aggregatzustand | fine crystals |
| pka | 10.33(at 25℃) |
| Farbe | White |
| Wichte | 2.17 |
| Geruch (Odor) | Odorless |
| Säure-Base-Indikators(pH-Indikatoren) | 8 - 9 |
| PH | 8.27(1 mM solution);8.25(10 mM solution);8.13(100 mM solution); |
| Wasserlöslichkeit | Soluble in water. Insoluble in alcohol. |
| maximale Wellenlänge (λmax) | λ: 260 nm Amax: 0.010 λ: 280 nm Amax: 0.01 |
| Merck | 14,7609 |
| BRN | 4535309 |
| Stabilität | Stable. |
| InChIKey | TYJJADVDDVDEDZ-UHFFFAOYSA-M |
| CAS Datenbank | 298-14-6(CAS DataBase Reference) |
| R-Sätze: | 22-35 |
| S-Sätze: | 24/25-45-36/37/39-26 |
| WGK Germany | 1 |
| RTECS-Nr. | FG1840000 |
| F | 3 |
| TSCA | Yes |
| HS Code | 28364000 |
| Toxizität | LD50 orally in Rabbit: > 2000 mg/kg |

