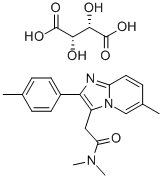
Zolpidem tartrate
Bezeichnung:Zolpidem tartrate
CAS-Nr99294-93-6
Englisch Name:Zolpidem tartrate
CBNumberCB7451799
SummenformelC23H27N3O7
Molgewicht457.48
MOL-Datei99294-93-6.mol
Zolpidem tartrate physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 196 °C |
| storage temp. | 2-8°C |
| Löslichkeit | Slightly soluble in water, sparingly soluble in methanol, practically insoluble in methylene chloride. |
| Aggregatzustand | Solid |
| Farbe | White to Off-White |
| BCS Class | 1 |
| CAS Datenbank | 99294-93-6(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | Xi,T,F |
| R-Sätze: | 36/37/38-39/23/24/25-23/24/25-11 |
| S-Sätze: | 26-36-45-36/37-16-7 |
| HS Code | 2933996500 |
| Giftige Stoffe Daten | 99294-93-6(Hazardous Substances Data) |


