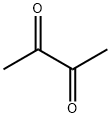
Diacetyl
Bezeichnung:Diacetyl
CAS-Nr431-03-8
Englisch Name:2,3-Butanedione
CBNumberCB3853625
SummenformelC4H6O2
Molgewicht86.09
MOL-Datei431-03-8.mol
Synonyma
Butandion
Diacetyl
Dimethylglyoxal
Diacetyl physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -4--2 °C |
| Siedepunkt | 88 °C(lit.) |
| Dichte | 0.985 g/mL at 20 °C |
| Dampfdichte | 3 (vs air) |
| Dampfdruck | 52.2 mm Hg ( 20 °C) |
| FEMA | 2370 | DIACETYL |
| Brechungsindex | n |
| Flammpunkt | 45 °F |
| storage temp. | Store at +2°C to +8°C. |
| Löslichkeit | 200g/l |
| Aggregatzustand | Liquid |
| Farbe | Clear yellow |
| Geruch (Odor) | at 1.00 % in propylene glycol. strong butter sweet creamy pungent caramel |
| Geruchsart | buttery |
| Explosionsgrenze | 2.4-13.0%(V) |
| Odor Threshold | 0.00005ppm |
| Wasserlöslichkeit | 200 g/L (20 ºC) |
| Merck | 14,2966 |
| Kennzeichnung gefährlicher | F,Xn |
| R-Sätze: | 11-20/22-38-41-36/38-20/21/22-37/38 |
| S-Sätze: | 9-16-26-37/39-36/37/39-39 |
| RIDADR | UN 2346 3/PG 2 |
| WGK Germany | 2 |
| RTECS-Nr. | EK2625000 |
| F | 13 |
| Selbstentzündungstemperatur | 365 °C |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29141990 |
| Giftige Stoffe Daten | 431-03-8(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 1580 mg/kg (Jenner) |




