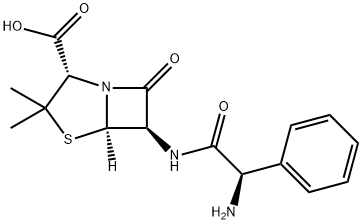
Ampicillin
Bezeichnung:Ampicillin
CAS-Nr69-53-4
Englisch Name:Ampicillin
CBNumberCB2247346
SummenformelC16H19N3O4S
Molgewicht349.4
MOL-Datei69-53-4.mol
Synonyma
Ampicillin
Ampicillin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 198-200 °C (dec.)(lit.) |
| alpha | D23 +287.9° (c = 1 in water) |
| Siedepunkt | 201.8°C (rough estimate) |
| Dichte | 1.2794 (rough estimate) |
| Brechungsindex | 1.6320 (estimate) |
| storage temp. | 2-8°C |
| Löslichkeit | NH4OH 1 M: 50 mg/mL, clear, colorless |
| Aggregatzustand | solid |
| Farbe | White to Pale Yellow |
| pka | 2.5 (COOH)(at 25℃) |
| Optische Aktivität | +287.923 (c 1.0, H2O) |
| Biologische Quelle | microbial |
| Wasserlöslichkeit | 13.9g/L(25 ºC) |
| Merck | 13,591 |
| JECFA Number | 85 |
| BRN | 1090925 |
| Stabilität | Stable, but may be moisture sensitive. Incopmpatible with strong oxidizing agents. |
| InChIKey | AVKUERGKIZMTKX-NJBDSQKTSA-N |
| Kennzeichnung gefährlicher | Xn,T |
| R-Sätze: | 36/37/38-42/43-61 |
| S-Sätze: | 22-26-36/37-45-53 |
| WGK Germany | 2 |
| RTECS-Nr. | XH8425000 |
| F | 10-23 |
| Selbstentzündungstemperatur | 301°C |
| HS Code | 29212900 |
| Giftige Stoffe Daten | 69-53-4(Hazardous Substances Data) |
| Toxizität | LD50 oral in mouse: > 5gm/kg |

