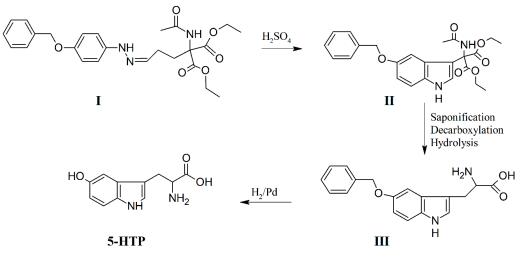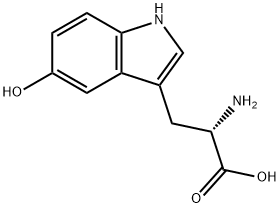
5-Hydroxytryptophan
Bezeichnung:5-Hydroxytryptophan
CAS-Nr56-69-9
Englisch Name:5-Hydroxytryptophan
CBNumberCB0110022
SummenformelC11H12N2O3
Molgewicht220.22
MOL-Datei56-69-9.mol
Synonyma
5-Hydroxytryptophan
5-Hydroxytryptophan physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 298-300°C |
| Siedepunkt | 361.16°C (rough estimate) |
| Dichte | 1.484 |
| Brechungsindex | 1.5200 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| Löslichkeit | DMSO (Slightly), Methanol (Slightly) |
| Aggregatzustand | solid |
| pka | 2.22±0.10(Predicted) |
| Farbe | Off-White to Pale Beige |
| Wasserlöslichkeit | Soluble in water (4 mg/ml at 25°C), methanol. |
| Merck | 14,4847 |
| BRN | 88199 |
| InChIKey | LDCYZAJDBXYCGN-UHFFFAOYSA-N |
| LogP | -0.292 (est) |
| CAS Datenbank | 56-69-9(CAS DataBase Reference) |
| EPA chemische Informationen | Tryptophan, 5-hydroxy- (56-69-9) |
| S-Sätze: | 26 |
| WGK Germany | 3 |
| RTECS-Nr. | YN7100000 |
| PackingGroup | III |
| Giftige Stoffe Daten | 56-69-9(Hazardous Substances Data) |