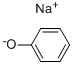
Sodium benzenolate
- русский язык имя
- английское имяSodium benzenolate
- CAS №139-02-6
- CBNumberCB7306886
- ФормулаC6H5NaO
- мольный вес116.09
- EINECS205-347-3
- номер MDLMFCD00013134
- файл Mol139-02-6.mol
химическое свойство
| Температура плавления | >300℃ |
| плотность | 0,898 g/cm3 |
| RTECS | SM8780000 |
| Fp | 28°C |
| температура хранения | below 5° C |
| растворимость | DMSO (Slightly, Sonicated), Methanol (Slightly) |
| форма | Solid |
| цвет | Pale Yellow to Pale Brown |
| Удельный вес | 0.898 |
| Растворимость в воде | Very soluble in water, soluble in alcoholSoluble in water, acetone and alcohol. |
| Чувствительный | Hygroscopic |
| Стабильность | Hygroscopic |
| Справочник по базе данных CAS | 139-02-6(CAS DataBase Reference) |
| FDA 21 CFR | 310.545 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 4NC0T56V35 |
| Система регистрации веществ EPA | Sodium phenate (139-02-6) |
| UNSPSC Code | 12352303 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 34 | |||||||||
| Заявления о безопасности | 26-36/37/39-45 | |||||||||
| РИДАДР | 3286 | |||||||||
| TSCA | No | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 2907110000 | |||||||||
| Банк данных об опасных веществах | 139-02-6(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
Sodium benzenolate химические свойства, назначение, производство
Химические свойства
Sodium phenate, or sodium phenoxide, NaOC6H5, is a white crystalline substance that easily dissolves in water and alcohol. It decomposes when exposed to carbon dioxide in the air. It is produced by reacting sodium hydroxide solution (not carbonate) with phenol and subsequently evaporating the mixture. Sodium phenate is commonly used in the production of sodium salicylate.Использование
As a general disinfectant, either in solution or mixed with slaked lime, etc., for toilets, stables, cesspools, floors, drains, etc.; for the manufacture of colorless or light-colored artificial resins, many medical and industrial organic Compounds and dyes; as a reagent in chemical analysis. Pharmaceutic aid (preservative).Подготовка
Sodium phenoxide was freshly prepared by adding sodium metal to phenol (0.45 g, 4.8 mmol) dissolved in diethyl ether (15 cm3), under an atmosphere of dry nitrogen. This was refluxed with (11) (1.0 g, 2.8 mmol) for 23 h. Following evaporation of the ether, water (20 cm3) was added and the mixture extracted into DCM. The DCM solution was dried (MgS04) and evaporated to give a solid (1.2 g). Fractional sublimation and recrystallisation from acetonitrile gave recovery of 2,4,6-tribromo-3,5-difluoropyridine (0.8 g, 80%) and 3-phenoxy-5-fluoro-2,4,6-tribromopyridine (0.2 g, 20%), m.p. 100±1 OC (Found: C, 31.3; H, 1.1; N, 2.9. C11H5Br3FNO requires C, 31.0; H, 1.2; N, 3.3%); IR spectrum no. 15; mass spectrum no. 15; nmr spectrum no. 19.Общее описание
A white to reddish colored solid in the form of crystalline rods. Used as an antiseptic and in organic synthesis.Реакции воздуха и воды
Decomposes in air. Soluble in water. In both cases, a corrosive alkaline solution forms that is a strong irritant to skin and eyes.Профиль реактивности
Salts, basic, such as SODIUM PHENOLATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.Опасность
Strong irritant to skin and tissue.Профиль безопасности
Poison by subcutaneous route. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Na2O. See also PHENOL and SODIUM HYDROXIDE.Методы очистки
The ground powder is washed with Et2O, then heated at 60o/1mm for 12 to 24hours to remove any free phenol and solvent. [Kornblum & Lurie J Am Chem Soc 81 2710 1959, Beilstein 6 I 718.]Sodium benzenolate запасные части и сырье
сырьё
запасной предмет
- 2-БРОМЭТИЛФЕНИЛСУЛЬФИД
- (4-BROMOPHENOXY)ACETIC ACID ETHYL ESTER
- Хлорид 2-феноксипропионил
- Бромбензол
- 2,6-Diiodo-4-nitrophenol
- 4-ФЕНОКСИФТАЛОНИТРИЛ
- 1,1,2,2-Тетракис (4-гидроксифенил) этан
- Propoxybenzene
- 1-бром-3-феноксибензол
- Фенил пропаргил эфир
1of4
Sodium benzenolate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-371-66670886 | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-024-23769576 +86-15040101888 |
China | 254 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 |
Sodium benzenolate Обзор)
1of4