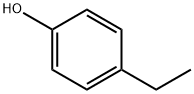
4-этилфенол
- английское имя4-Ethylphenol
- CAS №123-07-9
- CBNumberCB2106353
- ФормулаC8H10O
- мольный вес122.16
- EINECS204-598-6
- номер MDLMFCD00002393
- файл Mol123-07-9.mol
химическое свойство
| Температура плавления | 40-42 °C(lit.) |
| Температура кипения | 218-219 °C(lit.) |
| плотность | 1,011 g/cm3 |
| плотность пара | 4.2 (vs air) |
| давление пара | 0.13 mm Hg ( 20 °C) |
| FEMA | 3156 | P-ETHYLPHENOL |
| показатель преломления | 1.5330 |
| Fp | 213 °F |
| температура хранения | Keep in dark place,Sealed in dry,Room Temperature |
| растворимость | 4.9g/l |
| форма | Crystalline Mass or Crystals |
| пка | pK1:10.0 (25°C) |
| Удельный вес | 1.0123 (20℃) |
| цвет | White to brownish |
| Запах | at 1.00 % in dipropylene glycol. phenolic castoreum smoke guaiacol |
| Odor Type | smoky |
| Биологические источники | synthetic |
| Растворимость в воде | 4.9 g/L (25 ºC) |
| Номер JECFA | 694 |
| БРН | 1363317 |
| Стабильность | Stable. Incompatible with acid chlorides, acid anhydrides, oxidizing agents. |
| ИнЧИКей | HXDOZKJGKXYMEW-UHFFFAOYSA-N |
| LogP | 2.1 at 20℃ and pH7 |
| Surface tension | 54mN/m at 998g/L and 22℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | P-ETHYLPHENOL |
| Справочник по базе данных CAS | 123-07-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | AGG7E6G0ZC |
| Справочник по химии NIST | Phenol, 4-ethyl-(123-07-9) |
| Система регистрации веществ EPA | p-Ethylphenol (123-07-9) |
| UNSPSC Code | 41116107 |
| NACRES | NA.22 |
больше
| Коды опасности | Xi,C | |||||||||
| Заявления о рисках | 36/37/38-R36/37/38-34 | |||||||||
| Заявления о безопасности | 26-36-S36-S26-45-36/37/39 | |||||||||
| РИДАДР | UN 2430 8/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | SL4040000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29071900 | |||||||||
| Банк данных об опасных веществах | 123-07-9(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
4-этилфенол химические свойства, назначение, производство
Описание
4-Ethylphenol (4-EP) is a phenolic compound with the formula C8H10O. It is colorless or white needles with a powerful woody-phenolic and yet somewhat sweet odor and tends to be yellow on exposure to light. It is slightly solouble in water, solouble in alcohol, ether and benzene and carbon disulfide. It is found in arabica coffee and can be produced in wine and beer by the spoilage yeast Brettanomyces. It is the starting material for the production of 4-vinylphenol and of various antioxidants and can also be used as an intermediate for pharmaceuticals and dyes. It is an industrially versatile commodity chemical widely applied in the pharmaceutical and food industries.Химические свойства
4-Ethylphenol, also known as p-Ethylphenol, is a colorless or white crystalline compound. It can turn yellow when exposed to light. It has a powerful woody-phenolic, medicinal, yet somewhat sweet odor. Its melting point is 4143℃, boiling point is 218219°C, and flash point is 100°C. It has a relative density of 1.011 and a refractive index of 1.5239. It is slightly soluble in water and soluble in ethanol, ether, acetone, benzene, and carbon disulfide. It is a natural component found in foods such as egg fruit, European sour cranberry, pork, rum, whisky, and coffee.Вхождение
Reported found in yellow passion fruit, smoked fish, lean fish, beer, cognac, rum, whiskey, cider, sherry, grape wines and coffeeИспользование
4-Ethylphenol is suitable for use in the determination of 4-ethylguaiacol and 4-ethylphenol in the aroma of red wines using headspace-solid-phase microextraction method. It is a chemical reagent used in the synthesis of 5-HT1A receptor agonists endowed with antinociceptive activity in vivo. It is also used in the preparation of isopropylamides as potent and noncovalent proteosome inhibitors.Методы производства
4-Ethylphenol is produced industrially by sulfonation of ethylbenzene under mild (kinetically controlled) conditions and subsequent alkali fusion of the 4-ethylbenzenesulfonic acid obtained. The purity of the commercial product is 98%.Подготовка
4-Ethylphenol is prpared by catalytic reaction between phenol and ethylene or ethanol; by heating 8-chloro-3-ethylbenzene with NaOH in the presence of copper powder.Общее описание
4-Ethylphenol is a volatile phenol. It has been synthesized from p-coumaric acid by employing a synthetic medium containing Dekkera bruxellensis ISA 1791. It has been identified as an aroma compound in red wine.Опасность
Toxic material.Профиль безопасности
Poison by intravenous route. When heated to decomposition it emits acrid smoke and irritating vapors.Токсикология
4-Ethylphenol is a potentially toxic compound, capable of producing respiratory distress, cardiovascular collapse, shock, ventricular tachycardia, and coma in an adult. Liver, lung, central nervous system and renal injury may also occur. In case of exposure to eyes, irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes. Monitor for respiratory distress in case of inhalation exposure. Systemic manifestations of toxicity may include nausea, vomiting, diarrhea, dyspnea, tachypnea, pallor, and profuse sweating.Методы очистки
Non-acidic impurities are removed by passing steam through a boiling solution containing 1 mole of the phenol and 1.75 moles of NaOH (as an aqueous 10% solution). The residue is cooled and acidified with 30% (v/v) H2SO4, and the free phenol is extracted into diethyl ether. The extract is washed with water, dried with CaSO4 and the ether is evaporated. The phenol is distilled at 100mm pressure through a Stedman gauze-packed column (p 11). It is further purified by fractional crystallisation by partial freezing, and by zone refining, under N2 [Biddiscombe et al. J Chem Soc 5764 1963]. Alternatively purify it via the benzoate, as for phenol. The 4-nitrophenylbenzoate has m 80o (from EtOH). [Beilstein 6 H 470, 6 III 1663, 5 IV 3020.]использованная литература
[1] https://en.wikipedia.org/wiki/4-Ethylphenol[2] http://www.hmdb.ca/metabolites/HMDB29306
[3] https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~x8ypqu:1:cpp
[4] https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~x8ypqu:1:manf
[5] YING ZHANG Shaojun D Liangkun Long. Heterologous synthesis of 4-ethylphenol in engineered Escherichia coli[J]. Process Biochemistry, 2020, 96: Pages 157-164. DOI:10.1016/j.procbio.2020.06.003.
[6] WOHLFARTH C. Dielectric constant of 4-ethylphenol[C]. 1900: 0. DOI:10.1007/978-3-540-75506-7\_257.
[7] K H JONES D J H P W Trudgill. 4-Ethylphenol metabolism by Aspergillus fumigatus.[J]. Applied and Environmental Microbiology, 1994, 60 6: 1978-1983. DOI:10.1128/aem.60.6.1978-1983.1994.
4-этилфенол запасные части и сырье
сырьё
запасной предмет
1of2
4-этилфенол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18066069912 +86-18066069912 |
China | 18 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +8618629063126 | China | 435 | 58 | ||
| +8615318402391 | China | 809 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 |