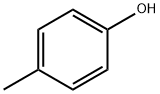
п-КРЕЗОЛ
- английское имяp-Cresol
- CAS №106-44-5
- CBNumberCB5453502
- ФормулаC7H8O
- мольный вес108.14
- EINECS203-398-6
- номер MDLMFCD00002376
- файл Mol106-44-5.mol
| Температура плавления | 32-34 °C(lit.) |
| Температура кипения | 202 °C(lit.) |
| плотность | 1.034 g/mL at 25 °C(lit.) |
| плотность пара | 3.72 (vs air) |
| давление пара | 1 mm Hg ( 20 °C) |
| FEMA | 2337 | P-CRESOL |
| показатель преломления | nD20 1.5395 |
| Fp | 193 °F |
| температура хранения | Store below +30°C. |
| растворимость | 20g/l |
| пка | 10.17(at 25℃) |
| форма | Crystalline Solid or Liquid |
| Удельный вес | 1.0341 (20/4℃) |
| цвет | Colorless to light yellow, may darken on exposure to light |
| Запах | at 0.10 % in dipropylene glycol. phenolic narcissus animal mimosa |
| Odor Type | phenolic |
| Биологические источники | synthetic |
| Порог?обнаружения?запаха? | 0.000054ppm |
| Пределы взрываемости | 1%(V) |
| Растворимость в воде | 20 g/L (20 ºC) |
| Чувствительный | Light Sensitive |
| Номер JECFA | 693 |
| Мерк | 14,2579 |
| БРН | 1305151 |
| констант закона Генри | 6.17 at 20.00 °C, 9.31 at 25.00 °C (dynamic equilibrium system-GC, Feigenbrugel et al., 2004a) |
| Пределы воздействия | NIOSH REL: TWA 2.3 ppm (10 mg/m3), IDLH 250 ppm; OSHA PEL: TWA 5 ppm (22 mg/m3); ACGIH TLV: TWA for all isomers 5 ppm (adopted). |
| Диэлектрическая постоянная | 5.6(21.0℃) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. Air and light-sensitive. Hygroscopic. |
| LogP | 1.94 |
| Справочник по базе данных CAS | 106-44-5(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | P-CRESOL |
| FDA 21 CFR | 172.515 |
| Рейтинг продуктов питания EWG | 7 |
| FDA UNII | 1MXY2UM8NV |
| Справочник по химии NIST | Phenol, 4-methyl-(106-44-5) |
| Система регистрации веществ EPA | p-Cresol (106-44-5) |
| UNSPSC Code | 77101502 |
| NACRES | NA.24 |
| Коды опасности | T,Xi | |||||||||
| Заявления о рисках | 24/25-34-39/23/24/25-23/24/25 | |||||||||
| Заявления о безопасности | 36/37/39-45-36/37 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 2.3 ppm (10 mg/m3) | |||||||||
| РИДАДР | UN 3455 6.1/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | GO6475000 | |||||||||
| F | 8 | |||||||||
| Температура самовоспламенения | 555 °C | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29071200 | |||||||||
| Банк данных об опасных веществах | 106-44-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 1.8 g/kg (Deichmann, Witherup) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H301+H311:Токсично при проглатывании или при контакте с кожей.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
п-КРЕЗОЛ химические свойства, назначение, производство
Химические свойства
Cresol is a mixture of three isomeric forms: o-, m-, and p-cresol. These compounds are slightly soluble in water. The m-isomer is a colorless or yellow liquid with characteristic odor. The p-cresol is a colorless to pink crystals with a characteristics phenol-like odor. The concentration at which the odor becomes detectable in water is 55 parts per billion (Buttery et al., 1988). Another study by Leonardos et al. (1969) reported an experimental odor threshold concentration of 1 parts per billion, which is higher than the 0.054 parts per billion reported by Nagata and Takeuchi (1990).Вхождение
Has been found in a score of essential oils including ylang ylang and oil of jasmine (Gildemeister & Hoffman, 1966).Использование
p-Cresol is utilized in the production of artificial resins, liquid crystal intermediates, disinfectants, fumigants, and as an industrial solvent. It is also involved in the manufacturing of Bupranolol, a non-selective beta blocker.Определение
ChEBI: P-cresol is a cresol that consists of toluene substituted by a hydroxy group at position 4. It is a metabolite of aromatic amino acid metabolism produced by intestinal microflora in humans and animals. It has a role as a uremic toxin, a human metabolite and an Escherichia coli metabolite.Методы производства
The cresols (cresylic acids) are methyl phenols and generally appear as a mixture of isomers. p-Cresol is a 4-methyl derivative of phenol and is prepared from m-toluic acid or obtained from coal tar or petroleum. Crude cresol is obtained by distilling “gray phenic acid” at a temperature of ~180–201°C. p-Cresol may be separated from the crude or purified mixture by repeated fractional distillation in vacuo. It can also be prepared synthetically by diazotization of the specific toluene or by fusion of the corresponding toluenesulfonic acid with sodium hydroxide.Подготовка
It can be prepared by fractional distillation of coal tar where it occurs together with the ortho- and para- isomers.Общее описание
Colorless solid with a tar like odor. Sinks and mixes slowly with water.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
p-Cresol is sensitive to heat. p-Cresol is also sensitive to light. p-Cresol is incompatible with strong oxidizers and strong alkalis. p-Cresol will attack some forms of plastics, coatings and rubber.Угроза здоровью
INHALATION: Irritation of nose or throat. EYES: Intense irritation and pain, swelling of conjunctiva and corneal damage may occur. SKIN: Intense burning, loss of feeling, white discoloration and softening. Gangrene may occur. INGESTION: Burning sensation in mouth and esophagus. Vomiting may result. Absorption by all routes may cause muscular weakness, gastroenteric disturbance, severe depression and collapse. Effects are primarily on central nervous system, edema of lungs, injury of spleen and pancreas may occur.Профиль безопасности
Poison by ingestion, skin contact, subcutaneous, intravenous, and intraperitoneal routes. A severe skin and eye irritant. Questionable carcinogen with experimental neoplastigenic data by itself and with 7,12-dirnethyl benz(a)anthracene. Combustible when exposed to heat or flame. Moderately explosive in the form ofvapor when exposed to heat or flame. To fight fire, use CO2, dry chemical, alcohol foam. See also other cresol entries and PHENOL.Возможный контакт
Cresol is used as a disinfectant and fumigant; as an ore flotation agent, and as an intermediate in the manufacture of chemicals, dyes, plastics, and antioxidants. A mixture of isomers is generally used; the concentrations of the components are determined by the source of the cresol.Канцерогенность
o-Cresol has been induced a few papillomas but no carcinomas in tumor studies.Метаболизм
p-Cresol is oxidized at the methyl group in both dogs and rabbits to yield ρ-hydroxybenzoic acid. In the rabbit up to 10% of oral doses of 0.25-0.5 g is excreted as free and conjugated p-hydroxybenzoic acid (Williams, 1959).Перевозки
UN2076 Cresols, liquid, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. UN3455 Cresols, solid, Hazard class: 6.1; Labels: 6.1- Poisonous materials, 8-Corrosive material.Методы очистки
It can be separated from m-cresol by fractional crystalisation of its melt. Purify it by distillation, by precipitation from *benzene solution with pet ether, and via its benzoate, as for phenol. Dry it under vacuum over P2O5. It has also been crystallised from pet ether (b 40-60o) and by conversion to sodium p-cresoxyacetate which, after crystallisation from water is decomposed by heating with HCl in an autoclave [Savard Ann Chim (Paris) 11 287 1929]. The 3,5-dinitrobenzoate (prepared with 3,5-dinitrobenzoyl chloride in dry pyridine, and recrystallised from EtOH or aqueous Me2CO) has m 189o. [Beilstein 6 II 2093.]Несовместимости
Vapors may form explosive mixture with air. Incompatible with strong acids; oxidizers, alkalies, aliphatic amines; amides, chlorosulfonic acid; oleum. Decomposes on heating, producing strong acids and bases, causing fire and explosion hazard. Liquid attacks some plastics and rubber. Attacks many metals.Утилизация отходов
Wastewaters may be subjected to biological treatment. Concentrations may be further reduced by ozone treatment. High concentration wastes may be destroyed in special waste incinerators.п-КРЕЗОЛ запасные части и сырье
сырьё
1of2
запасной предмет
- p-Tolyl phenylacetate
- 4-метиланизол
- Толтеродин
- 2,2-Dimethyl-3-(2-methylpropyl)cyclopropanecarboxylic acid p-(methoxymethyl)benzyl ester
- P-TOLYL ACETATE
- 2-бром-4-метилфенол
- 3,5-DIMETHYL-BENZOFURAN-2-CARBOXYLIC ACID
- 2-[3-[Bis(1-methylethyl)amino]-1-phenylpropyl]-4-methylphenol
- 2-[[4-[[4-[bis(2-hydroxyethyl)amino]-6-chloro-1,3,5-triazin-2-yl]amino]phenyl]azo]-p-cresol
- Mn(Ⅲ)salen epoxidation catalyst
- Этил-4-ethoxyphenylacetate
- 2-трет-Бутил-4-метилфенол
- 4-CHLOROMETHYL-6-METHYL-CHROMEN-2-ONE
- 4-гидроксибензальдегида
- Ethyl 3,5-dimethylbenzofuran-2-carboxylate ,95%
1of5
п-КРЕЗОЛ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 |
п-КРЕЗОЛ Обзор)
1of4