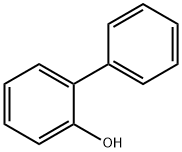
2-фенилфенол
- английское имя2-Phenylphenol
- CAS №90-43-7
- CBNumberCB0365303
- ФормулаC12H10O
- мольный вес170.21
- EINECS201-993-5
- номер MDLMFCD00002208
- файл Mol90-43-7.mol
| Температура плавления | 57-59 °C(lit.) |
| Температура кипения | 282 °C(lit.) |
| плотность | 1.21 |
| Плотность накопления | 600kg/m3 |
| давление пара | 7 mm Hg ( 140 °C) |
| показатель преломления | 1.6188 (estimate) |
| FEMA | 3959 | 2-PHENYLPHENOL |
| Fp | 255 °F |
| температура хранения | Store below +30°C. |
| растворимость | Soluble in ethanol, acetone, benzene,sodium hydroxide, chloroform, acetonitrile, toluene, hexane, ligroin, ethyl ether, pyridine, ethylene glycol, isopropanol, glycol ethers and polyglycols. |
| форма | Crystalline Flakes |
| пка | 10.01(at 25℃) |
| цвет | White |
| РН | 7 (0.1g/l, H2O, 20℃) |
| Запах | nearly wh. or lt. buff crystals, mild char. sweetish odor |
| Биологические источники | synthetic |
| Пределы взрываемости | 1.4-9.5%(V) |
| Растворимость в воде | 0.7 g/L (20 ºC) |
| Чувствительный | Hygroscopic |
| Номер JECFA | 735 |
| Мерк | 14,7304 |
| БРН | 606907 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, halogens. |
| ИнЧИКей | LLEMOWNGBBNAJR-UHFFFAOYSA-N |
| LogP | 3.18 at 22.5℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | O-PHENYLPHENOL |
| FDA 21 CFR | 175.105 |
| Справочник по базе данных CAS | 90-43-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 6-9 |
| FDA UNII | D343Z75HT8 |
| Предложение 65 Список | o-Phenylphenol |
| Код УВД | D08AE06 |
| МАИР | 3 (Vol. 73) 1999 |
| Справочник по химии NIST | o-Hydroxybiphenyl(90-43-7) |
| Система регистрации веществ EPA | 2-Phenylphenol (90-43-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi,N | |||||||||
| Заявления о рисках | 36/37/38-50-37/38/50-36 | |||||||||
| Заявления о безопасности | 22-61 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | DV5775000 | |||||||||
| Температура самовоспламенения | >520 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 9 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29071900 | |||||||||
| Банк данных об опасных веществах | 90-43-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 2.48 g/kg (Hodge) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H318:При попадании в глаза вызывает необратимые последствия.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
2-фенилфенол химические свойства, назначение, производство
Описание
2-phenylphenol(OPP) is a broad spectrum fungicide used to protect crops in storage. It is highly soluble in water, moderately voatile but is not expected to be persistent in the environment. OPP is more selective than other free phenols but does produce phytotoxic effects.Химические свойства
o-Phenylphenol is a white to buff-colored crystalline solid with a distinct odor. When heated to decomposition, it emits acrid smoke and irritating fumes.Использование
2-Phenylphenol is a agriculture fungicide and is no longer used as a food additive.Методы производства
OPP is produced as a by-product in the manufacture of diphenyl oxide or by aldol condensation of hexazinone.Определение
ChEBI: 2-Phenylphenol is a member of the class of hydroxybiphenyls that is biphenyl substituted by a hydroxy group at position 2. It is generally used as a post-harvest fungicide for citrus fruits. It has a role as an environmental food contaminant and an antifungal agrochemical. It derives from a hydride of a biphenyl.Подготовка
2-Phenylphenol can be recovered from the distillation residue of the process of phenol production via sulfonation. The phenol distillation residue contains about 40% of phenyl phenol with the other components including phenol, inorganic salts, water and so on. After vacuum distillation, the mixed phenyl phenol fraction is separated out with the vacuum being 53.3-66.7kPa. The temperature, started to be cut at 65-75 ℃ to until 100 ℃ above, but should not higher than 1345 ℃. Then take advantage of the solubility difference of ortho, p-hydroxy biphenyl in the trichlorethylene, the two are separated into pure product. The mixed material (mainly 2-hydroxy biphenyl and 4-hydroxy biphenyl) is heated to be dissolved in the trichlorethylene, after cooling, first precipitate out 4-hydroxy biphenyl crystal. After centrifuge filtration, dry to obtain 4-hydroxy biphenyl. The mother liquor was washed with a sodium carbonate solution, followed by dilute alkaline to make the 2-hydroxybiphenyl salt. After standing stratification, take the upper 2-hydroxybiphenyl sodium salt for dehydration under reduced pressure, namely, sodium salt products. The 2-hydroxybiphenylsodium salt is white to light red powder, being easily soluble in water with the solubility in 100g of water being 122g. The pH value of the 2% aqueous solution is 11.1-12.2. It is also easily soluble in acetone, methanol, soluble in glycerol, but insoluble in oil. The sodium salt of 2-hydroxy biphenyl, after acidification, can lead to the formation of 2-hydroxy biphenyl with both of them being food additives.прикладной
2-phenylphenol is remarkably versatile organic chemical products, widely used antiseptic, auxiliaries and surfactant synthesis of new plastics, resins and polymer materials in areas such as stabilizers and flame retardants. It is used for the post-harvest control of storage diseases of apples, citrus fruit, stone fruit, tomatoes, cucumbers and other vegetables. It is also used for the protection of textiles and timber and as a fungistat in water-soluble paints.Общее описание
Light lavender crystals or solid.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
2-Phenylphenol react as a weak organic acid. Exothermically neutralizes bases. May react with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides to generate flammable gas (H2) and the heat of the reaction may ignite the gas. Is sulfonated very readily (for example, by concentrated sulfuric acid at room temperature) in exothermic reactions. May be nitrated very rapidly. Nitrated phenols often explode when heated and also form metal salts that tend toward detonation by rather mild shock. Can react with oxidizing agents .Пожароопасность
2-Phenylphenol is combustible.Сельскохозяйственное использование
Fungicide, Disinfectant, Microbiocide: Used to make fungicides. Also used to make dye stuffs and rubber chemicals, but used primarily as a disinfectant cleaner. Registered for use in the U.S. and U.K.Торговое название
ANTHRAPOLE 73®; DOWCIDE-1®; Invalon OP®; KIWI LUSTR-277®; NECTRYL®; PREVENTOL-O Extra®; REMOL TRF®; TETROSIN OE®; TETROSIN OE-N®; TORSITE®; TUMESCAL OPE®Профиль безопасности
A poison by intraperitoneal route.Moderately toxic by ingestion and possibly other routes.An experimental teratogen. Other experimentalreproductive effects. Human mutation data reported.Severe eye and moderate skin irritant. Questionablecarcinogen wiВозможный контакт
o-Phenylphenol is used in the manufacture of plastics, resins, rubber, as Agricultural chemical, in making fungicides; as an intermediate in making dye stuffs and rubber chemicals; a germicide; used in food packaging.Канцерогенность
IARC classified SOPP as a B2 carcinogen in 1983, based on reports from Japan that high dietary levels of this sodium salt caused bladder tumors in male rats. Both sodium saccharin and sodium cyclamate also cause bladder tumors at high doses in male rats, but classification of these food additives as B2 carcinogens was recently rescinded by IARC at a meeting in 1998.The U.S. National Toxicology Program conducted a skin painting study with OPP in groups of 50 mice per sex. The OPP was applied as an acetone solution on 3 days per week for 2 years, both alone and as a promoter with DMBA. No skin neoplasms were observed in either sex treated with OPP alone, and there were no tumor enhancing or inhibiting effects when OPP and DMBA were given in combination.
Метаболический путь
2-Phenylphenol is not used on growing plants because it is too phytotoxic and there appears to be no information published on its metabolism in plants. Its widespread use as a preservative, disinfectant and fungistat on stored food (either by direct application or impregnated in packaging) requires studies on its environmental fate and metabolism in mammals. Several studies in mammals are available and the compound has been the subject of an evaluation by the UK MAFF Pesticide Safety Directorate (PSD); the results have been published (PSD, 1993). This evaluation was prompted by the discovery of bladder tumours in rats treated with high doses of the compound.2-Phenylphenol is also used as the sodium and potassium salts where water solublity is important. No information is available specifically on the latter. The metabolism of the free phenol and the sodium salt have been studied separately. Once absorbed into a cell, provided that internal pH control is maintained, the two forms should be indistinguishable.
Перевозки
UN3143 Dyes, solid, toxic, n.o.s. or Dye intermediates, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name RequiredМетоды очистки
Crystallise it from pet ether. [Beilstein 6 IV 4579.]Оценка токсичности
2-phenylphenol has a moderate to low toxicity to biodiversity. This substance has a low oral mammalian toxicity, is carcinogenic, a neurotoxin and recognised irritant. 2-Phenylphenol and its sodium salt have a low acute toxicity in mammals when administered orally, with LD50 values ranging from 600 to 3500 mg/kg of body weight.http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1340.htm
Деградация
2-Phenylphenol is readily degraded in surface waters and municipal waste mixtures, and the degradation is biologically mediated. In river water, radiolabelled 2- phenylphenol at concentrations ranging from 1.2 to 120 μg/litre was degraded to about 50% of the initial concentration in 1 week. The addition of mercuric chloride to inhibit biological activity reduced the decrease to only 10% after 30 days. In activated sludge, radiolabelled 2-phenylphenol at 9.6 mg/litre was degraded to 50% of the initial concentration in 24 h. 2-Phenylphenol therefore meets the criteria to be classified as readily biodegradable (FAO/WHO, 1999).Несовместимости
Strong bases, strong oxidizersУтилизация отходов
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.2-фенилфенол запасные части и сырье
2-фенилфенол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613393234347 | China | 2946 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| 010-60279497 | CHINA | 1803 | 55 | ||
| 0411-84820922 8613904096939 |
China | 304 | 57 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |
2-фенилфенол Обзор)
1of4