The role of 1,2,4-triazole
General description
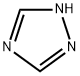
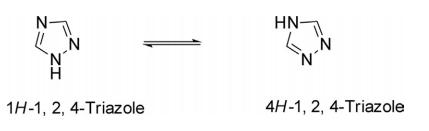
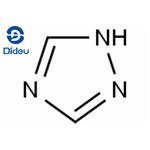
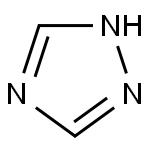
Triazole is an aromatic five membered heterocyclic compound. Its unique structure is conducive to the formation of a variety of non covalent bonds and is easy to promote the binding of proteins, enzymes and receptors, thus showing a wide range of biological pharmacological activities, such as anticancer, antispasmodic, antibacterial, anti HIV. In particular, 1,2,3-triazole has strong thermal stability and acid-base stability, and is not sensitive to redox, hydrolysis and enzymatic degradation. It is often used as a pharmacodynamic group in drug synthesis. 1,2,4-triazole is a chemical substance with molecular formula C2H3N3, molecular weight 69.07 and CAS No. 288-88-0. Colorless acicular crystal, soluble in water and ethanol. 1H-1,2,4-triazole is a 1,2,4-triazole. It is a tautomer of a 3H-1,2,4-triazole and a 4H-1,2,4-triazole.
Application and pharmacology
The product is used in the production of pesticides, pharmaceuticals (fluconazole), dyes and rubber additives, and also in the photoconductor of replication system. Intermediate of antifungal fluconazole.
1.During the post chemical mechanical polishing ( CMP) cleaning process of multilayer Cu interconnection for integrated circuits,it is necessary to remove the products and residues on the Cu sur face in the CMP process and protect the interconnection metal Cu and barrier layer metal from corrosion. The effects of 1,2,4-triazole ( TAZ) in the alkaline cleaning solution based on FA/O Ⅱ on the removal of abrasive particles adsorbed on the Cu surface and galvanic corrosion of Cu and Co were studied. The scanning electron microscope ( SEM) was used to detect the surface morphology and residue particles of the wafer after cleaning. The effects of different concentrations of TAZ in the cleaning solution on the cor rosion potential difference between Cu and Co and the corrosion current of them were studied by the electrochemical experiment.The experimental results show that TAZ can decrease the contact angle of Cu sur face,which is conducive to particle removal. The cleaning solution containing 25 mmol /L TAZ can re move most of the particles on the Cu surface.In addition,the cleaning solution containing 20 mmol /L TAZ can reduce the corrosion potential difference between Cu and Co to 0. 178 V,effectively inhibiting galvanic corrosion[1].
2. The recent advances of 1,2,4-triazole-quinoline/quinolone hybrids as potential anti-bacterial agents. The structure-activity relationship is also discussed for further rational development of 1,2,4-triazole hybrids higher potency against both drug-sensitive and drug-resistant pathogens. 1,2,4-Triazole, which can act as the isostere of amide, ester, carboxylic acid and other heterocycles, is common pharmacophore in many drugs. 1,2,4-Triazole derivatives possess a varied of pharmacological properties such as anti-tubercular, anti-fungal, anti-tumor and anti-bacterial activities due to 1,2,4-triazole moiety can exert various non-covalent interactions that are able to improve the solubility and the ability of binding to bimolecular targets. Moreover, several 1,2,4-triazole-based drugs such as fluconazole and ribavirin have already used in clinics for the treatment of various diseases. Thus, 1,2,4-triazole derivatives play an intriguing role in the development of new drugs[2].
Figure 1 the application and reserach of 1,2,4-Triazole
3.Hybrid molecules have the capacity to overcome drug cross resistance, represent promising candidates the development of new drugs. Hybridization of two or more anti-bacterial pharmacophores may provide new candidates with excellent potency against both drug-sensitive and drug-resistant bacteria. 1,2,4-Triazole and quinoline/quinolone derivatives possess promising in vitro and in vivo anti-bacterial activity, so hybridization of 1,2,4-triazole and quinoline/quinolone is rationale strategy to develop new anti-bacterial candidates.
Synthesis
Alkyl bromide and sodium azide were used as raw materials to prepare organic azides, and a series of 1,2,3-triazole small molecules were prepared by click chemistry. The structures of the six products were characterized by 1H NMR, 13C NMR and IR. the products were expected targets. Furthermore, the effect of different substituent structure on the reaction was discussed. Especially the introduction of drug molecule ethisterone, which is expected to provide a certain experimental basis for the application of triazole and its derivatives in biomedicine and other fields[3].
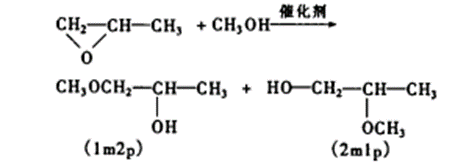
Figure 2 the synthesis route of 1,2,4-Triazole
As shown in Fig. 2, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.[3]
Toxicity
The 1,2,4‐triazole–naphthalimide hybrid39(MIC: >512 µg/ml, two‐fold serial dilution technique) was devoid of activity against MRSA, whereas incorporation of the substituted benzyl group into the N‐4 position of 1,2,4‐triazole could increase the anti‐MRSA activity, as evidenced by the fact that 1,2,4‐triazolium–naphthalimide hybrid40(MIC: 4.0–32 µg/ml, two‐fold serial dilution technique) showed an enhanced activity.[79]For hybrid40, the length of the alkyl linker between 1,2,4‐triazolium and naphthalimide moieties had a great influence on the activity, and extension of the carbon spacer decreased the activity. It was observed that hybrid40a(MIC: 4.0 µg/ml), which was as potent as chloromycin (MIC: 4.0 µg/ml) against MRSA, possessed a considerable activity (MIC: 1.0–16 µg/ml) againstS. aureus,B. subtilis,M. luteus,B. proteus,E. coli,P. aeruginosa,B. typhi,C. albicans, a n dC. mycoderma. This hybrid is a useful template for the development of broad‐spectrum antibacterial and antifungal agents. The 1,2,4‐triazole–berberine hybrids41a–d(MIC: 4.0–8.0 µg/ml, two‐fold serial dilution technique) exhibited a potential activity against S. aureus and MRSA, and the anti‐MRSA activity was equal to that of norfloxacin (MIC: 8.0 µg/ml) and 2–16 times superior to that of chloromycin (MIC: 16 µg/ml) and berberine (MIC: 128 µg/ml).
Reference
1.Wang Yazhen, Zhang Shihao, Tan Baimei, etc.: the role of inhibitor 1,2,4-triazole in Cu CMP post cleaning, semiconductor technology, No. 11, 2020, pp. 886-891.
2.Ge X. & Xu Z., "1,2,4‐Triazole hybrids with potential antibacterial activity against methicillin‐resistantStaphylococcus aureus," Archiv der Pharmazie, Vol.354, No.1(2021), p.2000223.
3.Xiao Ying, Wang Chaoyang, Chen Hancheng, etc.: synthesis and characterization of 1,2,3-triazole compounds, Guangdong chemical industry, No. 19, 2021, pp. 3-4.
Related articles And Qustion
See also
Lastest Price from 1,2,4-Triazole manufacturers
US $0.00-0.00/kg2025-11-27
- CAS:
- 288-88-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg
US $0.00/kg2025-11-25
- CAS:
- 288-88-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise