Synthesis, Properties, Chemical Reactivity of 1,2,4-Triazole
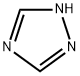
1,2,4-Triazole is a five-membered, π-excessive, aromatic nitrogen heterocycle, comprised of two carbon and three nitrogen atoms present at the 1-, 2-, and 4-positions of the ring. All the atoms in 1,2,4-triazoles are sp2 hybridized and have 6π electrons delocalized over the ring, responsible for its aromatic character. It is also known as s-triazole (symmetrical). 1,2,4-Triazole exists in two tautomeric forms known as 1H-1,2,4-triazole and 4H-1,2,4-triazole and it is very difficult to separate them due to their rapid interconversion.
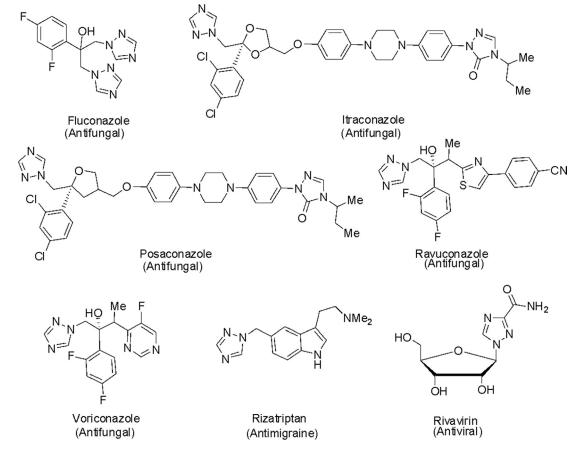
The diverse pharmacological activities of 1,2,4-triazoles as antifungal, antiviral, herbicidal, and catalase inhibitors induced deep interest to discover new entities for their broader applications. There are numerous 1,2,4-triazole-based drugs in clinical use for the treatment of various diseases. Some of the important drugs available in the market are depicted in the following scheme.
Physical Properties
Parent 1H-1,2,4-triazole is a white powder solid with an mp of 119–121°C, a bp of 260°C, a density of 1.13 g/cm3 , and is highly soluble in water. It is also soluble in alcohol, propanol, isopropanol, methyl acetate, and ethyl acetate. Its ionization potential is 10.00 eV; the dipole moment in the gas phase is 2.72 D and in dioxane it is 3.27 D.
UV (THF), λnm (ε): 216.5 (3.66).
1 H NMR (MeOH-d4 ), δ (ppm): C3 –H, 7.92; C5 –H, 8.85.
13C NMR (MeOH-d4 ), δ (ppm): C3 , 147.4; C5 , 147.4.
Synthesis
The parent 1H-1,2,4-triazole has been prepared by the reaction of hydrazine obtained in situ from the hydrolysis of 1,2-di(butan-2-ylidene)hydrazine with formamide at 170°C.
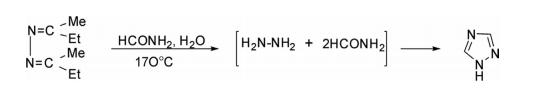
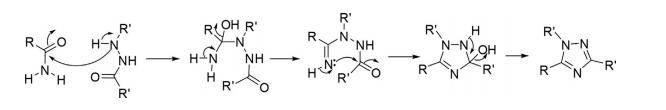
Pellizzari Synthesis
Amides on reaction with hydrazides form acyl amidrazone as an intermediate, which after intramolecular cyclization delivered 1,2,4-triazoles.
Einhorn-Brunner Synthesis
Acid-catalyzed condensation of formylacetamide with alkyl hydrazines delivered 1-alkyl-5-methyl-1,2,4-triazoles.
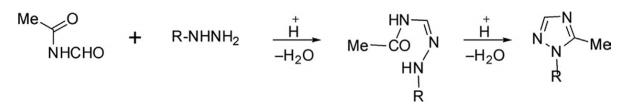
From Amidines
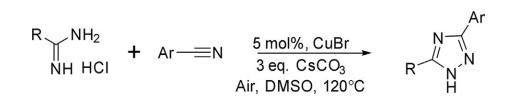
Amidines are considered good precursors for the synthesis of 1,2,4-triazole on reaction with different reagents and catalysts. 1,2,4-Triazoles have been synthesized by copper-catalyzed oxidative coupling of organic nitrile with amidine under atmospheric air in DMSO at 120°C.
Chemical Reactivity
Both carbon atoms in 1H-1,2,4-triazoles are π deficient because both are attached to electronegative nitrogen atoms and the electron density (0.744) at both carbon atoms is low and susceptible to nucleophilic substitution under mild conditions. It is a weak base and the pKa of 2.19 is for protonated species. The NH-protons in N-unsubstituted-1,2,4- triazoles are acidic in nature. The pKa of 1,2,4-triazoles is 10.26. The triazolium ions formed are also prone to nucleophiles. Electrophilic substitution takes place only at nitrogen atoms because of high electron density.
Electrophilic Substitution Reactions
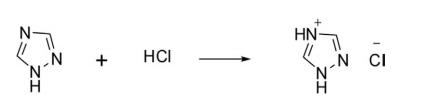
The parent 1H-1,2,4-triazole is readily protonated at position 4 in concentrated HCl to form triazolium chloride.
Metalation
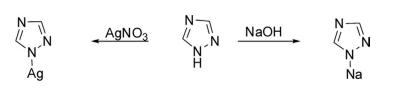
1H-1,2,4-Triazoles are easily metalated with NaOH, AgNO3 , and copper nitrate to form respective organometallic compounds.
Alkylation
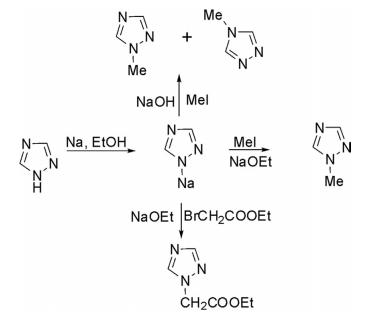
1H-1,2,4-Triazole is regioselectively alkylated at N1 when sodium ethoxide in ethanol is used as a base but alkylation in aqueous NaOH with methyl sulfate gave a mixture of 1-methyl- and 4-methyl-1,2,4-triazole. Alkylation with ethyl chloroacetate in sodium methoxide produced N1 -substituted products.
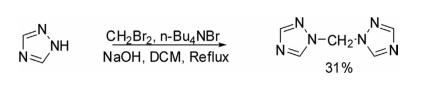
1H-1,2, 4-Triazole on reaction with dibromomethane using aqueous NaOH as a base and n-Bu4 NBr as a phasetransfer catalyst in DCM resulted in bis(1,2,4-triazol-1-yl)methane in 31% yield.
Related articles And Qustion
See also
Lastest Price from 1,2,4-Triazole manufacturers
US $0.00-0.00/kg2025-11-27
- CAS:
- 288-88-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg
US $0.00/kg2025-11-25
- CAS:
- 288-88-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise