Introduction of Propionic acid
General description
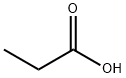
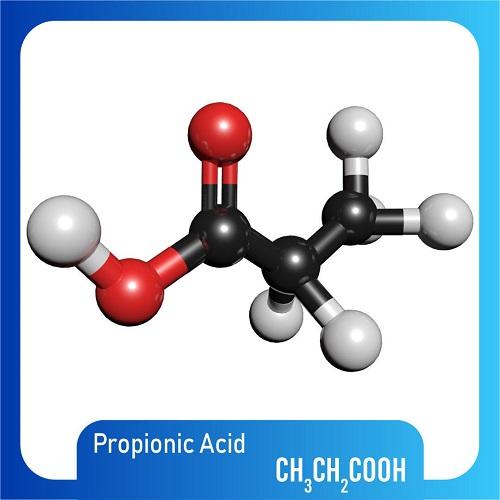
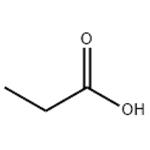
Propionic acid is a short-chain saturated fatty acid comprising ethane attached to the carbon of a carboxy group. It has a role as an antifungal drug. It is a short-chain fatty acid and a saturated fatty acid. It is a conjugate acid of a propionate. Mercaptopropionic acid (Propionic acid) is an organic substance with the chemical formula of c3h6o2s. It is a transparent liquid with strong sulfide smell, odor and toxicity. This product is the intermediate of fenalo, which is also used as stabilizer of PVC. Propionic acid is a colorless liquid with a sharp rancid odor. Produces irritating vapor. (USCG, 1999) Propionic acid is a short-chain saturated fatty acid comprising ethane attached to the carbon of a carboxy group. It has a role as an antifungal drug. It is a short-chain fatty acid and a saturated fatty acid. It is a conjugate acid of a propionate.[2] Solubility: soluble in water, ethanol, benzene, toluene, ether, chlorinated hydrocarbons and most other organic solvents.
Application and pharmacology
Propionic acid is an intermediate in the production of pharmaceutical fenarol, and can be also used in the preparation of antioxidants, hardeners, cross-linking agents, biochemical reagents, resin additives and catalysts. It is also the raw material for the production of PVC heat stabilizer ester tin. Propionic acid has a very broad market prospect. In this paper, the research progress in synthesis of 3-mercapto - propionic acid in recent years is reviewed, and the literature and the preliminary test results of our research group are summarized. Propionic acid is an important organic acid with wide industrial applications, especially in the food industry. It is currently produced from petrochemicals via chemical routes. Increasing concerns about greenhouse gas emissions from fossil fuels and a growing consumer preference for bio-based products have led to interest in fermentative production of propionic acid, but it is not yet competitive with chemical production. To improve the economic feasibility and sustainability of bio-propionic acid, fermentation performance in terms of concentration, yield, and productivity must be improved and the cost of raw materials must be reduced. These goals require robust microbial producers and inexpensive renewable feedstocks, so the present review focuses on bacterial producers of propionic acid and promising sources of substrates as carbon sources. Emphasis is placed on assessing the capacity of propionibacteria and the various approaches pursued in an effort to improve their performance through metabolic engineering. A wide range of substrates employed in propionic acid fermentation is analyzed with particular interest in the prospects of inexpensive renewable feedstocks, such as cellulosic biomass and industrial residues, to produce cost-competitive bio-propionic acid Sodium propionate is the sodium salt of propionic acid that exists as colorless, transparent crystals or a granular crystalline powder. It is considered generally recognized as safe (GRAS) food ingredient by FDA, where it acts as an antimicrobial agent for food preservation and flavoring agent. Its use as a food additive is also approved in Europe. Sodium propionate is is prepared by neutralizing propionic acid with sodium hydroxide. Sodium propionate was previously approved in Canada as an active ingredient in Amino-Cerv (used to treat inflammation or injury of the cervix).[1,2]
Synthesis
It is concluded that using acrylic acid and hydrogen sulfide as raw materials and ion exchange resin as catalyst to synthesize Propionic acid with high yield is an ideal synthesis route. It is prepared from acrylonitrile and thiourea. First react thiourea with hydrochloric acid at 40 ℃ for 0.5h, add acrylonitrile dropwise, react at 110-113 ℃ for 3h, cool to 40 ℃, add 4% sodium hydroxide solution to pH 11, dehydrogenate for 4h, and neutralize with hydrochloric acid at 30 ℃ to pH 1-2. Then add benzene to extract, recover benzene, carry out vacuum distillation, and collect the fraction at 125-140 ℃ to obtain the finished product. In addition, hydrogen sulfide and pyruvate or β- Propionic acid can also be prepared by propionolactone reaction.[3]
The related synthesis route of Propionic acid
Storage and Safety
Store in a cool and ventilated warehouse. Keep away from kindling and heat sources. It shall be stored separately from oxidant, reductant and alkali, and mixed storage shall not be allowed. Provide corresponding varieties and quantities of fire-fighting equipment. The storage area shall be equipped with leakage emergency treatment equipment and appropriate receiving materials.
Reference
1.Ammar E. M. & Philippidis G. P., "Fermentative production of propionic acid: prospects and limitations of microorganisms and substrates," Applied Microbiology and Biotechnology, Vol.105, No.16-17(2021), pp.6199-6213.
2.Mittal S., Malde A. & Selvam C. et al., "Synthesis and evaluation of S -4-(3-thienyl)phenyl-α-methylacetic acid," Bioorganic & Medicinal Chemistry Letters, Vol.14, No.4(2004), pp.979-982.
3.Wang Yanhua, Li Meng: "research progress in the preparation of 3-mercaptopropionic acid", chemistry and adhesion, 2021, issue 04, page 297-299.
You may like
Related articles And Qustion
See also
Lastest Price from Propionic acid manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 79-09-4
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons
US $0.00/kg2025-04-15
- CAS:
- 79-09-4
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons