1,2,4-Triazole: Overview, Antioxidant Activities and Toxicity
General Description
1,2,4-Triazole has garnered significant attention for their diverse pharmacological activities, including antimicrobial, antifungal, and antioxidant properties. These compounds have shown promise in combating oxidative stress-related diseases by neutralizing free radicals and mitigating their harmful effects. Various research studies have demonstrated the antioxidant activities of different 1,2,4-Triazole derivatives through assays such as ABTS, DPPH, and FRAP, highlighting their potential therapeutic applications. However, it is important to note that 1,2,4-Triazole also exhibits toxicity, particularly as an eye irritant and a reproductive toxin, emphasizing the need for caution in handling and exposure to these compounds.

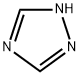
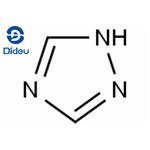
Figure 1. 1,2,4-Triazole
Overview
1,2,4-Triazole, a member of the triazole family, is a five-membered heterocyclic compound characterized by three nitrogen atoms within its structure, endowing it with a high electron density. Within the triazole family, there exist two isomeric forms based on the position of the nitrogen atoms: the 1,2,3-triazole and the 1,2,4-triazole, each exhibiting two tautomeric forms. First introduced by Boldwin in 1885, the term "triazole" specifically denotes a heterocyclic ring composed of two carbon and three nitrogen atoms (C2N3H3). Since its inception, triazole and its derivatives have garnered significant attention from researchers across various fields. Literature extensively documents the diverse pharmacological activities exhibited by these derivatives, including antimicrobial, antifungal, antibacterial, antitubercular, analgesic, anti-inflammatory, anticonvulsant, antidepressant, antimalarial, antiviral, and hypoglycemic properties. Notably, some derivatives also demonstrate tranquilizing effects and hold promise as anticancer agents. In the context of cellular health, 1,2,4-Triazole derivatives have garnered attention due to their potential antioxidant properties. In the face of various threats such as viral infections, bacterial pathogens, and oxidative stress, cells are vulnerable to damage, including DNA impairment. Oxygen-free radicals, including hydroxyl radicals, hydrogen peroxide, and superoxide anions, play a pivotal role in oxidative stress and the pathogenesis of numerous diseases. Antioxidants, including 1,2,4-Triazole derivatives, serve as crucial defense mechanisms against oxidative stress by neutralizing free radicals and mitigating their harmful effects. Therefore, exploring the antioxidant potential of 1,2,4-Triazole compounds holds promise for therapeutic interventions aimed at combating oxidative stress-related diseases. 1
Antioxidant Activities
The information about the presence of free radicals in biological materials was given for the first time about 70 years ago. Since then, numerous scientific studies have been conducted and the science of free radicals was introduced. Today we know that free radicals are by-products of enzymatic reactions occurring in the organism. They are produced during endogenous processes such as cell respiration, phagocytosis, biosynthesis, catalysis, and biotransformation. They can also be produced by exogenous processes (radiation, sunlight, heavy metals, bacteria, fungi, protozoa, and viruses). The overproduction of free radicals affects the aging processes, Oxidative Stress (OS) and takes part in the pathogenesis of various diseases. Among them are cancer, rheumatoid arthritis, neurodegenerative diseases: Alzheimer and Parkinson, pulmonary diseases, atherosclerosis, and DNA damage. Compounds with antioxidant activity are very important nowadays because they allow organisms to keep a balance between the production of free radicals and the speed of their neutralization in the body. Next to the natural antioxidants (flavonoids, carotenoids, vitamins, etc.), synthetic ones are also of great importance.
Among synthetic compounds with antioxidant activity are 1,2,4-triazoles and their derivatives. 1,2,4-Triazoles are heterocyclic compounds with three nitrogen atoms. Due to a broad spectrum of biological activities, these derivatives have been of interest to scientists for many years. Some of them are also used as drugs. The finding of new synthetic compounds with antioxidant features in the triazole group has become an important problem of medicinal chemistry.1
Toxicity
1,2,4-Triazole exhibits toxicity through various routes of exposure. It is documented as an eye irritant, causing redness and pain upon contact. Additionally, 1,2,4-Triazole can be absorbed through the skin and inhalation, leading to potential systemic effects. Animal studies suggest it may pose risks to human reproduction and development, qualifying as a reproductive toxin. This classification encompasses harm to reproductive function and developmental defects in offspring. Therefore, caution is advised in handling and exposure to 1,2,4-Triazole to mitigate potential adverse health effects, especially concerning reproductive health. 2
References:
[1] PACHUTA-STEC A. Antioxidant Activity of 1,2,4-Triazole and its Derivatives: A Mini-Review.[J]. Mini reviews in medicinal chemistry, 2022, 22 7. DOI:10.2174/1389557521666210401091802.[2] GESQUIERE J, SIEDEM C. 1,2,4‐Triazole[C]. 2006. DOI:10.1002/047084289X.RT160.PUB2.
You may like
Related articles And Qustion
See also
Lastest Price from 1,2,4-Triazole manufacturers
US $0.00-0.00/kg2025-11-27
- CAS:
- 288-88-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg
US $0.00/kg2025-11-25
- CAS:
- 288-88-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise