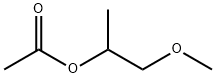
Introduction of 1-Methoxy-2-propyl acetate
General description
1-Methoxy-2-propyl acetate (PGMEA) is a material contains polar groups and non polar group , has good ability of dissolving and coupling, commonly used in solvent based coatings and screen printing ink.[1] Using dipropylene glycol methyl ether and acetic acid as raw material, under the catalysis of solid acid, via esterification reaction to generated crude product and its high purity PGMEA after distillation. Propylene glycol methyl ether acetate (PGMEA), also known as propylene glycol monomethyl ether acetate, with molecular formula of C6H12O3, is a colorless hygroscopic liquid with special smell. It is a non pollution solvent with multi-functional groups. It is mainly used as the solvent of ink, paint, ink, textile dye and textile oil agent, and also as the cleaning agent in the production of LCD. Flammable, may form explosive vapor / air mixture above 42 ° C. Propylene glycol methyl ether acetate (PGMEA) is an advanced solvent. Its molecule has both ether bond and carbonyl. Carbonyl forms the structure of ester and contains alkyl at the same time. In the same molecule, there are both non-polar and polar parts. The functional groups of these two parts not only restrict and repel each other, but also play their inherent roles. Therefore, it has a certain solubility for non-polar substances and polar substances.[2]
Application and pharmacology
1.Well dispersed silver nanoparticles (AgNPs) with narrow size distribution have been grown in organic solvent propylene glycol monomethyl ether acetate (PGMEA) by pulsed laser ablation techniques. The presence of AgNPs in PGMEA solvent gives rise to an enhancement of the absorption and nonlinear optical properties due to the surface plasmon resonance induced by AgNPs. The shape and density of the AgNPs have been estimated by fitting the absorption spectra with a given model, and the results also show that an additional laser irradiation treatment can improve the monodispersity of the AgNPs and their nonlinear optical properties. The synthesis of AgNPs in PGMEA will facilitate adding AgNPs into organic functional materials especially for photoresist to modify their optical properties.[3]
2.It is a high-grade industrial solvent with low toxicity and excellent performance. It has strong solubility for polar and non-polar substances. It is suitable for solvents of various polymers of high-grade coatings and inks, including aminomethyl ester, vinyl, polyester, cellulose acetate, alkyd resin, acrylic resin, epoxy resin and nitrocellulose. Among them. Propylene glycol methyl ether propionate is the best solvent in coatings and inks. It is suitable for unsaturated polyester, polyurethane resin, acrylic resin, epoxy resin, etc.[4]
Synthesis
1.A thorough kinetic investigation can be used to evaluate the potential utility of the reaction system and to support reaction engineering investigation for process development. For the kinetic resolution of 2-methyl-pantanol withPseudomonassp.,Indlekofer etal. (1993)successfully applied a kinetic model based on a Michaelis–Menten mechanism taking into account competitive inhibition of both enantiomer substrates. The model derived here has a similar basis. Competitive inhibition of theR-enantiomer product is considered additionally and the maximum velocity (Vmax,S) and affinity constant (Km,S) of the slower enantiomer are reduced to a single specificity constant (kS) due to the limited solubility of MPA in water. Due to the interesting properties of CAL-B, and the fact that a three-dimensional crystal structure is available (Uppenberg et al., 1995), this enzyme is often used in protein engineering to derive molecular models which predict the enantioselectivity of the enzyme towards a class of substrates like secondary alcohols. In case of (trans)-esterification reactions, the inter play of secondary alcohols with the active site of the enzyme and the resulting enantioselectivity has been studied by various authors.[3]
Figure 1 Scheme 1. CAL-B catalyzed enantioselective hydrolysis ofR/S-1-methoxy-2-propyl acetate catalyzed byCandida antarcticalipase B.
Storage
Store in a cool and ventilated warehouse; Keep away from kindling, heat source and anti-static. Transport as hazardous chemicals.
Reference
1.Liu Yanfang and Wang Shifa: Study on Synthesis of dipropylene glycol methyl ether acetate, Dalian, Liaoning, China, 2016.
2.Zogka A. G., Mellouki A. & Romanias M. N. et al., "Atmospheric Chemistry of 1-Methoxy 2-Propyl Acetate: UV Absorption Cross Sections, Rate Coefficients, and Products of Its Reactions with OH Radicals and Cl Atoms," The Journal of Physical Chemistry A, Vol.120, No.45(2016), pp.9049-9062.
3.Berendsen W. R., Gendrot G. & Resnick S. et al., "Kinetic modeling of lipase catalyzed hydrolysis of (R/S)-1-methoxy-2-propyl-acetate as a model reaction for production of chiral secondary alcohols," Journal of Biotechnology, Vol.121, No.2(2006), pp.213-226.
4.Shi H., Wang C. & Zhou Y. et al., "Silver Nanoparticles Grown in Organic Solvent PGMEA by Pulsed Laser Ablation and Their Nonlinear Optical Properties," Journal of Nanoscience and Nanotechnology, Vol.12, No.10(2012), pp.7896-7902.
You may like
Related articles And Qustion
See also
Lastest Price from 1-Methoxy-2-propyl acetate manufacturers

US $0.00/kg2025-05-26
- CAS:
- 108-65-6
- Min. Order:
- 230kg
- Purity:
- 99%
- Supply Ability:
- 20000

US $10.00/KG2025-04-21
- CAS:
- 108-65-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt