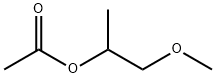
Unveiling 1-Methoxy-2-propyl Acetate: A Versatile Chemical for Modern Applications
Introduction
1-Methoxy-2-propyl acetate (PMA) is an organic acid ester compound, which is a colorless liquid with a sweet ether-like odor. PMA has a unique molecular structure consisting of a non-polar part and a polar group. Therefore, PMA has a good solubility for both polar and non-polar solvents. In addition, it has the characteristics of good thermal stability, high foaming, low viscosity change and corrosiveness, and is widely used in industries such as coatings, detergents, printing and dyeing, pesticides and pluronic polymers.

Figure 1 Characteristics of 1-Methoxy-2-propyl acetate
Uses
1-Methoxy-2-propyl acetate is a multifunctional organic solvent. In the industrial field, PMA is used as a solvent for paints, inks, varnishes, cleaners, coatings, and ink removers, especially for polyisocyanates contained in coatings. In the agricultural field, PMA is used as an inert ingredient in non-food pesticide products. In cosmetics or personal care products, it is used as a solvent in the formulation. In addition. PMA is also used in atmospheric chemistry research.
The rate coefficients for the reactions of OH and Cl with PMA in the gas phase were measured using absolute and relative methods. The kinetic study on the OH reaction was conducted in the temperature (263–373) K and pressure (1–760) Torr ranges using the pulsed laser photolysis-laser-induced fluorescence technique, a low pressure fast flow tube reactor-quadrupole mass spectrometer, and an atmospheric simulation chamber/GC-FID. The derived Arrhenius expression is kPMA+OH(T) = (2.01 ± 0.02) × 10–12 exp[(588 ± 123/T)] cm3 molecule–1 s–1. The absolute and relative rate coefficients for the reaction of Cl with MPA were measured at room temperature in the flow reactor and the atmospheric simulation chamber, which led to k(Cl+PMA) = (1.98 ± 0.31) × 10–10 cm3 molecule–1 s–1. GC-FID, GC-MS, and FT-IR techniques were used to investigate the reaction mechanism in the presence of NO. The products formed from the reaction of PMA with OH and their yields were methyl formate (80 ± 7.3%), acetic acid (50 ± 4.8%), and acetic anhydride (22 ± 2.4%), while for Cl reaction, the obtained yields were 60 ± 5.4, 41 ± 3.8, and 11 ± 1.2%, respectively, for the same products. The UV absorption cross section spectrum of PMA was determined in the wavelength range 210–370 nm. The study has shown no photolysis of PMA under atmospheric conditions.
Synthesis
1-Methoxy-2-propyl acetate (PMA) is prepared by continuous esterification reaction using propylene glycol methyl ether and acetic acid as raw materials. The specific reaction steps are as follows: a two-stage fixed bed continuous esterification reaction is carried out at an esterification temperature of 70 to 150 °C using a solid acid catalyst; water produced in the esterification reaction is removed by azeotropic distillation. The product of the esterification reaction is then distilled, and finally the excess propylene glycol methyl ether and acetic acid are removed or recovered.
References:
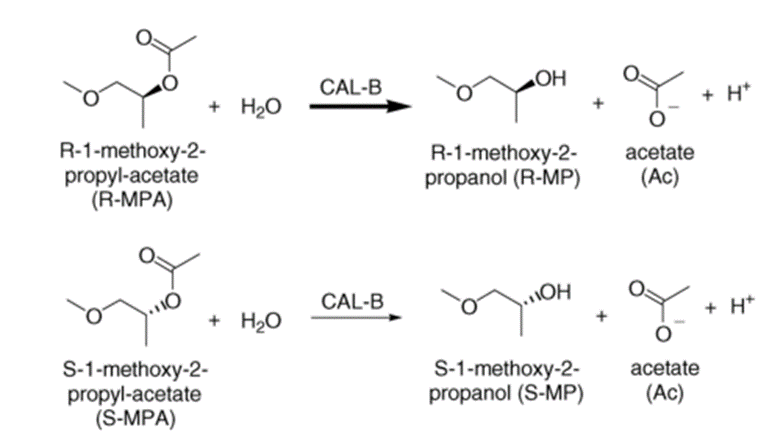
[1] W.R. BERENDSEN . Kinetic modeling of lipase catalyzed hydrolysis of (R/S)-1-methoxy-2-propyl-acetate as a model reaction for production of chiral secondary alcohols[J]. Journal of biotechnology, 2006, 121 2: 109-290. DOI:10.1016/j.jbiotec.2005.07.006.[2] YUN LI. Solubilities of carbon dioxide in 2-methoxyethyl acetate, 1-methoxy-2-propyl acetate and 3-methoxybutyl acetate[J]. Journal of Chemical Thermodynamics, 2014, 74: 1-290. DOI:10.1016/j.jct.2014.01.019.
[3] ANTONIA G. ZOGKA. Atmospheric Chemistry of 1-Methoxy 2-Propyl Acetate: UV Absorption Cross Sections, Rate Coefficients, and Products of Its Reactions with OH Radicals and Cl Atoms[J]. The Journal of Physical Chemistry A, 2016, 120 45: 8923-9130. DOI:10.1021/acs.jpca.6b08757.
Related articles And Qustion
Lastest Price from 1-Methoxy-2-propyl acetate manufacturers

US $0.00/kg2025-05-26
- CAS:
- 108-65-6
- Min. Order:
- 230kg
- Purity:
- 99%
- Supply Ability:
- 20000

US $10.00/KG2025-04-21
- CAS:
- 108-65-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt