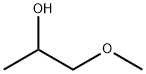
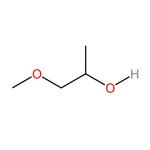
Introduction of 1-methoxy-2-propanol
General description
1-methoxy-2-propanol appears as a colorless liquid. Flash point near 89°F. Less dense than water. 1-Methoxy-2-propanol (M2P) is finding increasing industrial use as a less toxic alternative to the short-chained ethylene glycol ethers. Like most glycol ethers, M2P is readily absorbed through the skin and biological monitoring is therefore appropriate in assessing occupational exposure.
Application and pharmacology
It is mainly used as solvent, dispersant and diluent, as well as fuel antifreeze, extractant, etc. Used as solvent of nitrocellulose, compounding agent of brake oil and detergent, etc. Widely used in coatings and cleaners. It can be used as the active solvent of water-based coatings; Active solvent and coupling agent of solvent based printing ink; Solvent for ballpoint pens and pens; Coupling agents and solvents for household and industrial cleaners, derusting agents and hard surface cleaners; Solvents for agricultural pesticides; Mixed with propylene glycol n-butyl ether for glass cleaner formula[1].
The determination of free M2P(1-methoxy-2-propanol) in urine. The method involves solvent extraction, gas chromatography mass spectrometry and is sensitive (detection limit 1 pmol/l), specific and reproducible (intra- and inter-assay coefficients of variation 5% and 9%, respectively). A human volunteer study, involving six volunteers, was also conducted. Volunteers were exposed to 100 ppm M2P for 8 h (the occupational exposure standard in the UK) including a 30-min break. Post-exposure levels of free M2P in urine were found to reach up to 110 pmol/l. Levels of M2P were also monitored in blood (maximum 103 pmol/l) and exhaled air samples (up to 252 nmol/l). The volunteer study showed that M2P is rapidly excreted in urine with a half-life of less than 2.6 h.[2]
Synthesis
Propanediol monomethyl ether is the most important of these compounds. It is obtained by ring opening addition reaction of propylene oxide and methanol. Generally, the so-called propanediol monomethyl ether is a mixture of two isomers, methoxy propanediol and methoxy propanediol. Such products can meet the use requirements in most cases, but in some special synthesis reactions, such as the synthesis of cationic resin used in cathodic electrophoretic coating, the mass fraction is required to be more than because the existence of middle primary warp group interferes with the normal progress of the reaction, And the mass fraction of water is required to be below. Domestic propylene glycol monomethyl ether often can not meet such high requirements, and the general mass fraction is about. The reason is that the selectivity of the catalyst used in production is low. At present, the high content of cathodic electrophoretic coatings in China still depends on import. The finished product is obtained by the ring opening addition reaction of excess methyl alcohol and propylene oxide in the presence of catalyst, and then de intoxication and distillation. The reaction formula is as follows[3]:
Figure 1 the synthesis route of 1-methoxy-2-propanol
Toxicity
Contact irritates skin, eyes and mucous membranes. Prolonged exposure to vapors may cause coughing, shortness of breath, dizziness and intoxication. Vapors heavier than air. Used as a solvent and as an antifreeze agent. The oral LD50 of rats was 6.6g/kg. The skin irritation is not obvious, but the toxic dose can be absorbed through the skin. The main manifestations of animal poisoning were inhibition and incomplete anesthesia. Half of the rats died when they were exposed to steam concentration of 40.18g/m3 for 5 ~ 6 hours. the impact of 1-methoxypropanol-2 (MEP) for the stimulation of an inflammatory response in human respiratory mucosa, we exposed 22 primary cell cultures of nasal respiratory epithelia of healthy individuals to MEP concentrations at the level of the German MAK-value (100ppm) and to the 10-fold concentration (1000ppm). After 4 and 24h we analyzed the transcription of TNF-a, IL-1, IL-6, IL-8, MCP-1, GMCSF, Cox-1 and Cox-2 by quantitative PCR as well as the release of the respective cytokines by ELISA. At both MEP concentrations we observed a significant increase of TNF-a, IL-1, IL-6- and Cox-2-transcripts after 4 h. After 24 h cytokine transcription of TNF-a, IL-1 and IL-6 was normalized, but Cox-2 remained elevated. On the protein level IL-1. as well as granulocyte macrophages colony stimulating factor (GM-CSF) were decreased after 4 h or 24 h and uniquely IL-8 levels were increased after 4 h. Our data suggest that MEP induces the transcription. of genes encoding proinflammatory cytokines and mediators but largely not translation of those. Considering thesein vitrodata, existing exposure limits seem to be safe with respect to inflammatory responses of the upper respiratory tract. However, the effects of long-term exposures to MEP should be watched closely.[4,5]
Storage
Store in a cool and ventilated warehouse; Keep away from kindling, heat source and anti-static. Transport as hazardous chemicals.
Reference
1.Jones K., Dyne D. & Cocker J. et al., "A biological monitoring study of 1-methoxy-2-propanol: analytical method development and a human volunteer study," The Science of the total environment, Vol.199, No.1(1997), pp.23-30.
2.Lafita C., Penya-roja J. M. & Gabaldón C., "Anaerobic removal of 1-methoxy-2-propanol under ambient temperature in an EGSB reactor," Bioprocess and Biosystems Engineering, Vol.38, No.11(2015), pp.2137-2146.
3.Wang Weiqiang, Gu Haining, Li Xiaoling: Study on the synthesis of 2-aminopropanol, Zhejiang chemical industry, 2010, No. 01, pp. 15-17.
4.Brieger J., Muttray A. & Jung D. et al., "Early stress response of human nasal respiratory epithelia after exposure to 1-methoxypropanol-2," Toxicology Letters, Vol.177, No.2(2008), pp.138-143.
5.Laitinen J., Liesivuori J. & Savolainen H., "Biological monitoring of occupational exposure to 1-methoxy-2-propanol," J Chromatogr B Biomed Sci Appl, Vol.694, No.1(1997), pp.93-98.
You may like
Related articles And Qustion
Lastest Price from 1-Methoxy-2-propanol manufacturers
US $25.00/kg2025-11-23
- CAS:
- 107-98-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 200kg

US $0.00/kg2025-05-26
- CAS:
- 107-98-2
- Min. Order:
- 230kg
- Purity:
- 99.9%
- Supply Ability:
- 20000