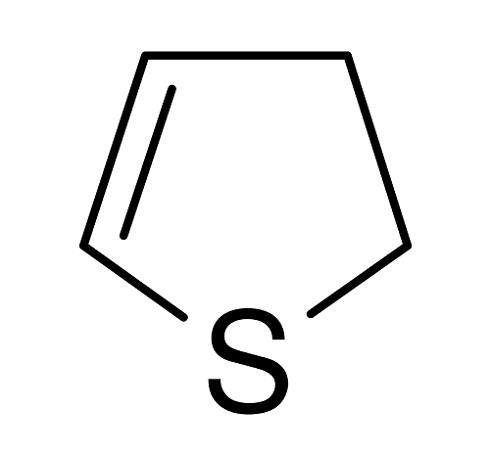
Synthesis of 2,5-Dihydrothiophene
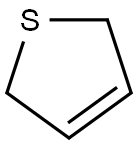
2,5-Dihydrothiophene is a nonaromatic, partially saturated, sulfur-containing five-membered heterocycle, comprised of four carbon atoms and one sulfur atom with a double bond between the C3 and C4 carbon atoms. It is also known as 3-thiolene. The rich synthetic potential of this ring system commenced more than half a century ago but the chemistry has not been developed extensively.
Physical Properties
The parent 2,5-dihydrothiophene is a liquid with a bp of 122°C and an mp of –50.3°C. It is soluble in most of the organic solvents.
Synthesis
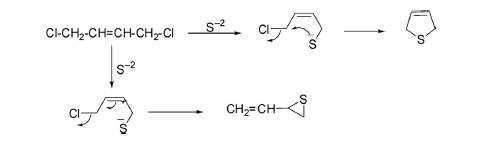
2,5-Dihydrothiophene has been synthesized by the condensation of 1,4-dichlorobut-2-ene with sodium sulfide in DMSO at 35–38°C together with vinylthirane. The pathway of cyclization is demonstrated in the following scheme.
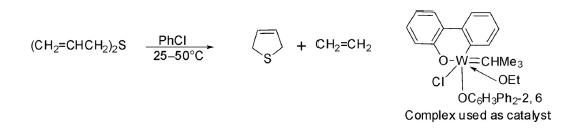
Diallyl sulfide has been transformed into 2,5-dihydrothiophene425 together with ethylene in the presence of a molybdenum- or tungsten-containing complex as catalyst in chlorobenzene at 25–50°C under vacuum.
Chemical Reactivity
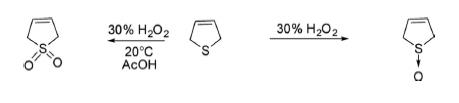
Oxidation 2,5-Dihydrothiophene oxidized with 30% H2O2 at low temperature provided 2,5-dihydrothiophene sulfoxide in 48% yields. However, hydrogen peroxide oxidation in acetic acid at 20°C for 24 h and thereafter boiling for 3 h gave 2,5-dihydrothiophene-1,1-dioxide.
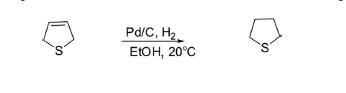
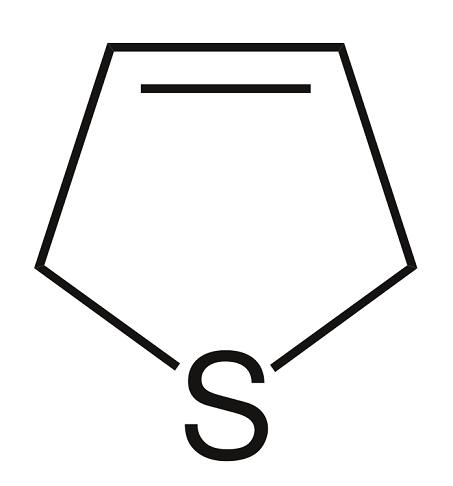
Hydrogenation of 2,5-dihydrothiophene over Pd/C in ethanol at 20°C for 12 min produced tetrahydrothiophene.