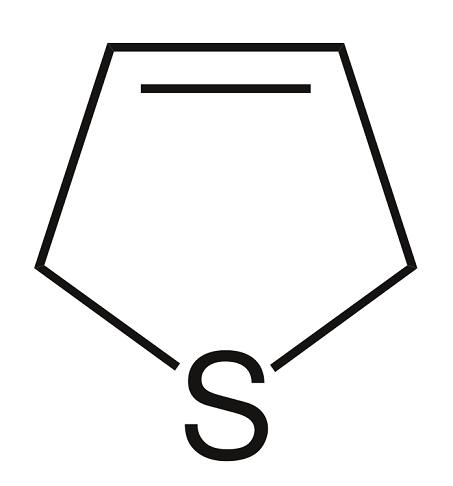
Chemical Reactivity of 2,5-Dihydrothiophene
The parent 2,5-dihydrothiophene is a liquid with a bp of 122°C and an mp of –50.3°C. It is soluble in most of the organic solvents.
Chemical Reactivity
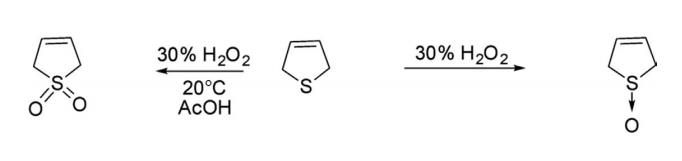
1.Oxidation 2,5-Dihydrothiophene oxidized with 30% H2O2 at low temperature provided 2,5-dihydrothiophene sulfoxide in 48% yields. However, hydrogen peroxide oxidation in acetic acid at 20°C for 24 h and thereafter boiling for 3 h gave 2,5-dihydrothiophene-1,1-dioxide.
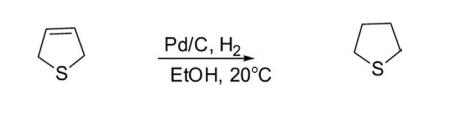
2. Reduction Hydrogenation of 2,5-dihydrothiophene over Pd/C in ethanol at 20°C for 12 min produced tetrahydrothiophene.
3. Addition of Thiols Addition of thiols to 2,5-dihydrothiophenes takes place at high temperature in an autoclave at 200°C to yield 3-alkylthiotetrahydrothiophene.

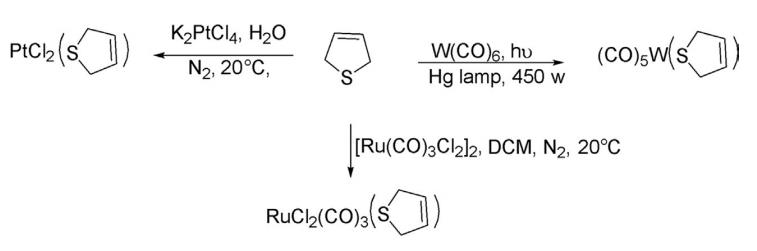
4. Reaction With Metal Complexes 2,5-Dihydrothiophene readily forms complexes on reaction with W(CO)6 , [Ru(CO)3 Cl2]2 , and K2 PtCl4 at room temperature to form various complexes.
5. Interconversion 2,5-Dihydrothiophene on heating at 300°C in the presence Re/Al2O3 isomerized to 2,3-dihydrothiophene in 21.8% yield.

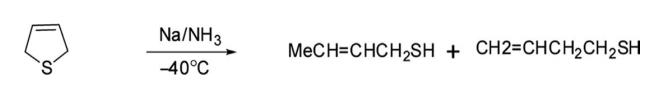
6. Ring-Opening Reaction Reduction of 2,5-dihydrothiophene with sodium in liquid ammonia in methanol gave a mixture of 2-butanethiol and 3-butanethiol as ring-opened compounds.
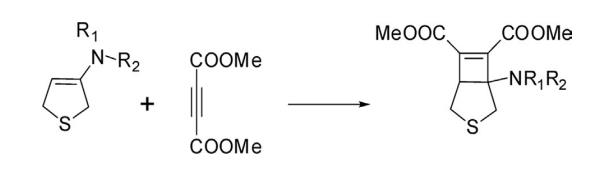
7. Cycloaddition Reactions 2,5-Dihydrothiophene having an enamine structural feature undergoes cycloaddition reactions with active dienophile. Thus a reaction of 3-dialkylamino-2,5-dihydrothiophene with dimethyl acetylene dicarboxylate gave a bicyclic compound, dimethyl 1-(dialkylamino)-3-thiabicyclo [3.2.0]hept-6-ene-6,7-dicarboxylate.

![270-82-6 Benzo[c]thiopheneStructureSynthesis](/NewsImg/2022-01-28/6377897911205665289585131.jpg)