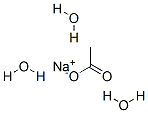
The review of sodium acetate trihydrate
Introduction
Sodium acetate trihydrate (SAT) is a hydrated salt with a phase transition temperature of 58°C and a latent heat value of 266kJ/kg. Due to its stable phase transition temperature, high latent heat of phase transition, non-toxicity, non-corrosion and good economy, The application is more extensive. However, as an inorganic phase change material, it also has the problems of large undercooling, poor phase separation and poor circulation effect. Choi Wenlong et al. used the nucleating agent method, vibration method and stirring method to eliminate the supercooling degree of sodium acetate trihydrate through experimental methods [1]. By adding different kinds of additives and nucleating agents, Wang Zhiping found that adding 5% disodium hydrogen phosphate dodecahydrate and 3% sodium carboxymethyl cellulose to sodium acetate trihydrate can effectively make the material annealed. cooling down to 2.02°C and prevent phase separation [2]. TAKAHIRO et al. added 1% sodium pyrophosphate as a nucleating agent to sodium acetate trihydrate, and the supercooling degree was reduced to less than 5 °C. After multiple cycle experiments, the material still had good heat storage performance [3].
Supercooling characteristics of sodium acetate trihydrate
The chemical formula of Sodium acetate trihydrate is NaCH3COO.3H20. Sodium acetate trihydrate is a promising heat storage material (m.p:58°C, heat of fusion, AHf~s:252 kJ/ kg (60.3 cal/g)[1]. This salt melts incongruently, producing anhydrous NaCH3COO. Besides, the salt can supercool heavily. Consequently, two problems of this hydrate, supercooling and phase separation, must be solved before practical use as a latent heat storage material. Supercooling characteristics of NaCH3COO. 3H20 are similar to those of Na2SEO3.5H20 and CaC12.6H20. During nucleation, the chemical potential of water must increase, as the water molecules rearrange themselves into the surroundings of the solid hydrate, because the chemical potential of water in crystalline state, Ixc, is higher than that in the supercooled melt, Ixm. The difference of the chemical potential (A IXH20 = IXc -- IX,.) may be considered a rough measure of the barrier to nucleation. Infrared spectroscopic characteristics of these molten hydrates have been clarified by us recently[4].
Application of Sodium acetate trihydrate in composite phase change materials
The composite phase change material was formed by expanded graphite (EG) with porous mesh structure and sodium acetate trihydrate (SAT) as the substrate [5]. The effects of EG on the properties of SAT were studied by using scanning electron microscope, temperature data logger, differential scanning calorimeter, and Hot Disk thermal constant analyzer etc. The results showed that the EG could not reduce the degree of supercooling of SAT, but it could largely improve the thermal conductivity of the material. EG had little effect on the latent heat value and phase transition temperature of SAT. EG could reduce the phase separation of SAT and improve its durability. Compared to the pure SAT, the degree of supercooling of composite PCM (7% EG+1% disodium hydrogen phosphate + SAT) was reduced by 49℃. Thermal conductivity of the composite PCM was doubled. Latent heat and phase transition temperature of the composite PCM were basically the same. After 50 cycles, the change of latent heat of composite PCM was kept within 1.4%, which had great
potential in thermal storage. As the picture 1 showed, there is ET/SAT composite material on SEM.
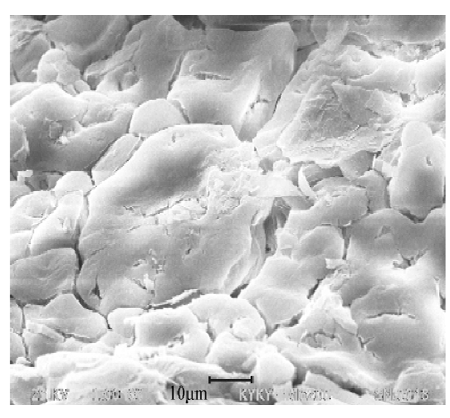
Picture 1 EG/SAT composite material [5]
Reference
Related articles And Qustion
See also
Lastest Price from Sodium acetate trihydrate manufacturers

US $1200.00-1100.00/ton2025-08-11
- CAS:
- 6131-90-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $1.00/KG2025-04-21
- CAS:
- 6131-90-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt