tert-Butyldimethylsilyl chloride: Synthesis, application and metabolism
General description
tert-Butyldimethylsilyl chloride (TBDMSCL) is often used as a protect agent in organic synthesis. The hydroxyl functionality has been found to be effectively protected by transformation to the silyl ether by reaction with tert-Butyldimethylsilyl chloride. In the synthesis of organic compounds, protection groups are used extensively. Protection groups are used to mask specific functionality which then allows other transformations to be affected in the molecule. After the intended transformation is carried out, the protected functionality is then regenerated by removal of the protecting group. As hydroxyl protectant in organic synthesis, derivated reagents are used for analysis and preparation. tert-Butyldimethylsilyl chloride reacts with alcohols to form silane and is used to synthesize prostaglandins, certain antibiotics, lovastatin and simvastatin. The appearance of tert-Butyldimethylsilyl chloride is as follows:

Figure 1 Appearance of tert-Butyldimethylsilyl chloride.
Synthesis
tert-Butyldimethylsilyl chloride can be synthesized by the previous work [1]. Use of Cu(I)Cl and KCN as a Mixed Catalyst To a 125 ml 3 neck flask fitted with a condenser and thermocouple under argon was added 15 ml THE. To this solution was added 0.042 g (0.4683 mmoles) copper (I) chloride and 0.030 g (0.4683 mmoles) potassium cyanide followed by addition of 6.04 g (0.0468 moles) dimethyldichloro silane. To this mixture held at 25°C. was added dropwise 30 ml of a 19 wt % (0.0468 moles) solution of t-butyl magnesium chloride in THE over 20 minutes. After addition was complete heated reaction to 60°C. for 4 hr. Reaction mixture was then cooled to 25°C. followed by addition of 25 ml of heptane. Solid magnesium chloride which precipitated out of the reaction mixture was then removed by filtration.
The resulting solution contained 6.42 g of tert-Butyldimethylsilyl chloride (91% yield) with only trace quantities of other identifiable impurities. IR 3478 (OH), 2942 (CH), 1779 and 1720 (C=O phth), 1254 (Si-CH3), 1122 (Si-O-C aliph), 1150-980 (C-O pyranosyl), 837 (Si-O-CH3 def), 719 (aromatic) cm-1. CP/MAS 13C NMR δ -5.75 (CH3Si), 18.39 (C4°), 25.81 (CH3 tert-butyl), 56.15 (C-2), 63.64 (C-6), 71.06 (C-3), 75.58 (C-5), 83.52 (C-4), 99.21 (C-1), 123.28, 132.52 (aromatic carbons), 167.76 (Phth C=O) ppm.
Application
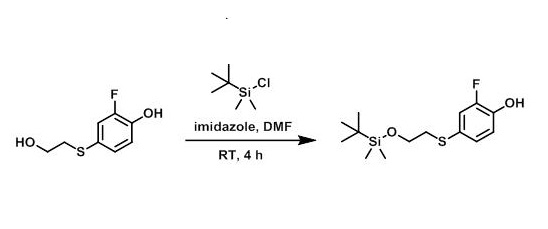
tert-Butyldimethylsilyl chloride can be used as a hydroxyl protectant. Corey and Venkateswarlu have described the use of tert-Butyldimethylsilyl chloride (in DMF at 35 "C in the presence of imidazole) for protecting the hydroxyl groups of a number of alcohols of interest in the synthesis of prostaglandins [2]. (a) TBDMSCL reacts preferentially with the 5'-hydroxyl group; (b) the reaction is rapid even at room temperature and the extent of the reaction and distribution of products depends on the amount of imidazole present; (c) even in the presence of excess TBDMSCL reaction occurs preferentially with hydroxyl groups and not with amino groups; (d) the TBDMS group is stable to phosphorylation conditions; and (e) the TBDMS group can be removed with (n-butyl),NF in tetrahydrofuran (THF) as described by Corey and Venkateswarlu without affecting other acid or base labile protecting groups on the nucleoside.
Metabolism
The metabolism of tert-Butyldimethylsilyl chloride in vitro and in vivo was studied in the previous work [3]. Uptake of tert-Butyldimethylsilyl chloride by human CEM cells is drug concentration-dependent and increased proportionally with increasing initial extracellular tert-Butyldimethylsilyl chloride concentrations up to 20 pg/mL. Within 6 hr of incubation, the cells were almost completely saturated with the test compound; further incubation up to 72 hr did not markedly increase the intracellular concentration of the compound. Upon intravenous bolus administration of tert-Butyldimethylsilyl chloride to mice at 0.75 mg/kg, it was rapidly cleared from the plasma in a mono-exponential manner (half-life: 22 min; distribution volume: 9.5 L/kg; total body clearance: 17.8 L/hr/kg). tert-Butyldimethylsilyl chloride mainly accumulated in the lungs, followed by the heart, kidney and liver. Significant amounts of different metabolites of tert-Butyldimethylsilyl chloride were detected in most tissues, the liver, kidney and spleen being the organs that showed the most extensive metabolism.
References
[1]Sims et al. Preparation of substituted silanes via organometallic catalysts mediated reaction of alkyl magnesium compounds with halosilane. U.S., 6429327, 06 Aug 2002.
[2]Ogilvie et al. The tevt-Butyldirnethylsilyl Group as a Protecting Group in Deoxynucleosides. Canadian Journal of Chemistry, 2011, 51: 3799.
[3]Jan Balzarini et al. Metabolism and pharmacokinetics of the anti-HIV-1-specific inhibitor [1-[2′,5′-Bis-O-(tert-butyldimethylsilyl)-β-d-ribofuranosyl]-3-N-methyl-thymine]-3′-spiro-5″-(4″-amino-1″,2″-oxathiole-. Biochemical Pharmacology, 1993, 46(1): 69-77.
You may like
Related articles And Qustion
See also
Lastest Price from tert-Butyldimethylsilyl chloride manufacturers

US $1.00-1.00/KG2025-09-12
- CAS:
- 18162-48-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $19.90/kg2025-04-21
- CAS:
- 18162-48-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt