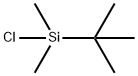
What is tert-Butyldimethylsilyl chloride?
Introduction
Reagent Uses
TBS Protection(TBS-Cl) Examples:
Example 1
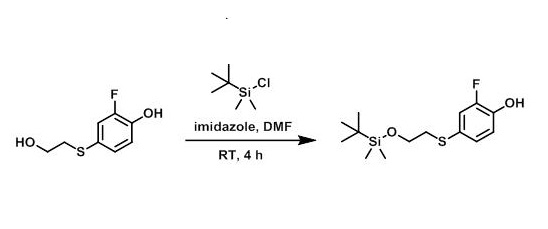
To a solution of the SM (985 mg, 5.24 mmol), imidazole (371 mg, 5.30 mmol), and DMF (5 mL) was added portionwise TBSCl (814 mg, 5.24 mmol). The reaction mixture was stirred at RT for 4 h. The mixture was concentrated in vacuo, diluted with H2O, and extracted with EtOAc (3x). The combined organics were washed with brine, dried (MgSO4), and concentrated to provide the product as an orange oil which was used without further purification. [1.43 g, 90%]
Example 2
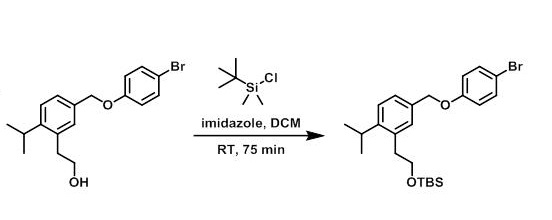
To a solution of the SM (1.2 g, 3.4 mmol), imidazole (0.70 g, 10 mmol), and dry DCM (33 mL) was added TBSCl (0.77 g, 5.1 mmol) under N2 at RT. The reaction mixture was stirred at RT for 75 min. The mixture was quenched with H2O and extracted with DCM. The layers were separated and the org layer was washed with H2O, brine, dried (MgSO4), and concentrated to provide the product as a colorless oil which was carried to the next step without further purification. [1.69 g, 100%]
Example 3
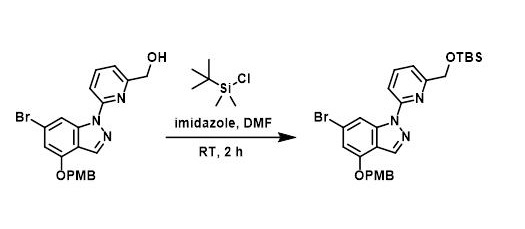
To a mixture of the SM (2.35 g, 5.36 mmol) and imidazole (729 mg, 10.72 mmol) in DMF (30 mL) at RT was added TBSCl (1.21 g, 8.04 mmol). The reaction mixture was stirred at RT for 2 h. The mixture was diluted with H2O (300 mL) and extracted with EtOAc (3 x 100 mL). The combined organics were washed with brine (100 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel column chromatography (5:1 PE/EtOAc) to provide the product as a yellow solid. [2.34 g, 79%]
Example 4
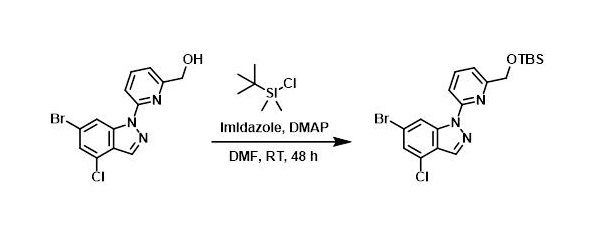
A mixture of the SM (0.750 g, 2.22 mmol), imidazole (0.302 g, 4.43 mmol), TBSCl (0.401 g, 2.66 mmol), and DMAP (0.01 g, 0.08 mmol) in DMF (6.86 mL) was stirred at RT for 48 h. The mixture was diluted with DCM, washed with NaHCO3, and concentrated. The residue was purified by silica gel chromatography (40 g silica, EtOAc/heptane) to provide the product as a clear solid. [600 mg]
You may like
Related articles And Qustion
See also
Lastest Price from tert-Butyldimethylsilyl chloride manufacturers

US $1.00-1.00/KG2025-09-12
- CAS:
- 18162-48-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $19.90/kg2025-04-21
- CAS:
- 18162-48-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt