Di-tert-butyl dicarbonate: Application, synthesis and toxicity
General description
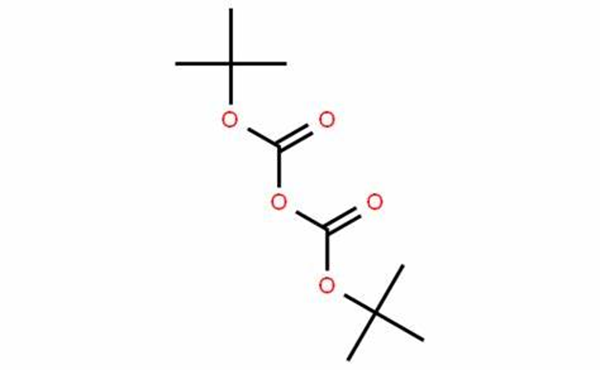
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids (e.g., trifluoroacetic acid). Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group. The appearance of Di-tert-butyl dicarbonate is as follows:

Figure 1 Appearance of Di-tert-butyl dicarbonate.
Synthesis
The Di-tert-butyl dicarbonate was successfully synthesized according to the previous work [1]. In 500 ml flask equipped with stirring device, thermometer, gas inlet tube and dropping funnel and it was replaced with nitrogen, after that, sodium t-butoxide 37.6 g (0.39 mol) and 233 ml hexane were charged. To this mixture at -10 ~10 °C, under stirring, carbon dioxide 11.2 L (0.5 mol) was bubbled over 3 hours. Then, the slurry mixture was obtained above, N-octyl pyridinium chloride 4.2 g (0.019 mol) and pyridine 4.2 g (0.053 mol) and t-butanol 0.8 g (0.01 mol) were added at -8 °C, after stirring for 30 minutes at the same temperature, at the same temperature methane sulfonyl chloride 23.5 g (0.21 mol) was added dropwise over 1 hour, it was incubated for 3 hours at 0 ~ 5 °C and the mixture was stirred. After completion of the reaction, 80 ml of 8% sulfuric acid was added to the reaction mixture, after stirring for 30 minutes, it was allowed to stand, the liquid was separated.
The resulting organic layer was washed sequentially with 5% aqueous solution of sodium bicarbonate and water and concentrated under reduced pressure at 35 ~ 40 °C, Di-tert-butyl dicarbonate 35.1 g (yield 81.5%) as white powder was obtained. The purity was analyzed by gas chromatography, the purity was 99.1%.
Application
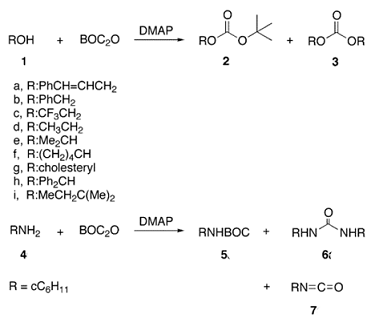
Di-tert-butyl dicarbonate can be used in the protection and deprotection of amines. The Boc group can be added to the amine under aqueous conditions using Di-tert-butyl dicarbonate in the presence of a base such as sodium bicarbonate. Protection of the amine can also be accomplished in acetonitrile solution using 4-dimethylaminopyridine (DMAP) as the base [2]. Removal of the Boc in amino acids can be accomplished with strong acids such as trifluoroacetic acid neat or in dichloromethane or with HCl in methanol. A complication may be the tendency of the t-butyl cation intermediate to alkylate other nucleophiles; scavengers such as anisole or thioanisole may be used.Selective cleavage of the N-Boc group in the presence of other protecting groups is possible when using AlCl3. Besides, the synthesis of 6-acetyl-1,2,3,4-tetrahydropyridine, an important bread aroma compound, starting from 2-piperidone was accomplished using Di-tert-butyl decarbonate [3]. The first step in this reaction sequence is the formation of the carbamate from the reaction of the amide nitrogen with boc anhydride in acetonitrile using DMAP as a catalyst. Di-tert-butyl dicarbonate also finds applications as a polymer blowing agent due to its decomposition into gaseous products upon heating [4].
Toxicity
Di-tert-butyl dicarbonate is hepatotoxic [5]. Di-tert-butyl dicarbonate (0 or 250 mg/kg/day) was given to male and female young intact and castrated rats by gavage for 28 days. In intact rats, relative liver weight increased to more than two times that of the control in males, while the rate of change was less than 10% in females. On histopathology, hypertrophy of hepatocytes was observed in males but not in females. In castrated rats, an approximately 40% increase in the relative liver weight was found only in males, and no histopathological changes in the liver were detected in either sex. The gender-related difference was also determined in preweaning rats administered Di-tert-butyl dicarbonate at 0, 250, or 500 mg/kg/day by gavage from postnatal days 4 to 21. Blood biochemical changes, including increases in the levels of AST, ALT, and ALP, 80–95% increase in the relative liver weight and histopathological changes in the liver, such as hypertrophy and single cell necrosis of hepatocytes, were observed at both doses in both sexes.
References
[1]Honda et al. Preparation of di-tert-butyl dicarbonate. Jpn. Kokai Tokkyo Koho, 2002097172, 02 Apr 2002.
[2]Yochai Basel; Alfred Hassner (2000). Di-tert-butyl Dicarbonate and 4-(Dimethylamino)pyridine Revisited. Their Reactions with Amines and Alcohols. J. Org. Chem. 65: 6368–6380.
[3]T. J. Harrison; G. R. Dake (2005). An Expeditious, High-Yielding Construction of the Food Aroma Compounds 6-Acetyl-1,2,3,4-tetrahydropyridine and 2-Acetyl-1-pyrroline. J. Org. Chem. 70 (26): 10872–10874.
[4]Wirth, D. (8 April 2020). Highly Expandable Foam for Lithographic 3D Printing. ACS Appl. Mater. Interfaces. 12 (16): 19033–19043.
[5]Hirata-Koizumi et al (2008). Gender-Related Difference in the Toxicity of Ultraviolet Absorber 2-(3′,5′-Di-tert-butyl-2′-hydroxyphenyl)-5-chlorobenzotriazole in Rats. 3(31): 383-398.
You may like
Related articles And Qustion
See also
Lastest Price from Di-tert-butyl dicarbonate manufacturers

US $0.00-0.00/kg2025-09-26
- CAS:
- 24424-99-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $50.00-10.00/kg2025-09-02
- CAS:
- 24424-99-5
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg